Depleting extracellular vesicles from fetal bovine serum alters proliferation and differentiation of skeletal muscle cells in vitro
- PMID: 27038912
- PMCID: PMC4818850
- DOI: 10.1186/s12896-016-0262-0
Depleting extracellular vesicles from fetal bovine serum alters proliferation and differentiation of skeletal muscle cells in vitro
Abstract
Background: Fetal bovine serum (FBS) contains a wide range of growth factors, hormones, vitamins, amino acids, fatty acids and trace elements required for cell growth. It was shown that animal sera contain also extracellular vesicles (EVs) with important biological properties; thus we wondered whether EVs present in FBS would influence muscle cell phenotype. EVs were removed from sera by ultracentrifugation (18 h). C2C12, L6 and human primary myoblasts, were grown either in classical media (CM) or in EVs-depleted media. Differentiation was induced by replacing the culture medium either with CM or EV-depleted media. qRT-PCR of relevant genes and miRNA involved in proliferation, differentiation, energy metabolism and EVs formation and secretion were performed.
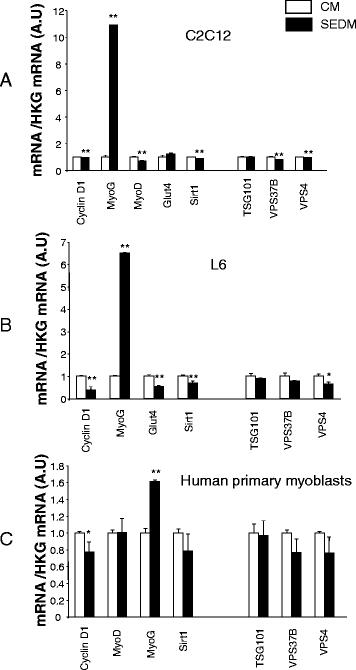
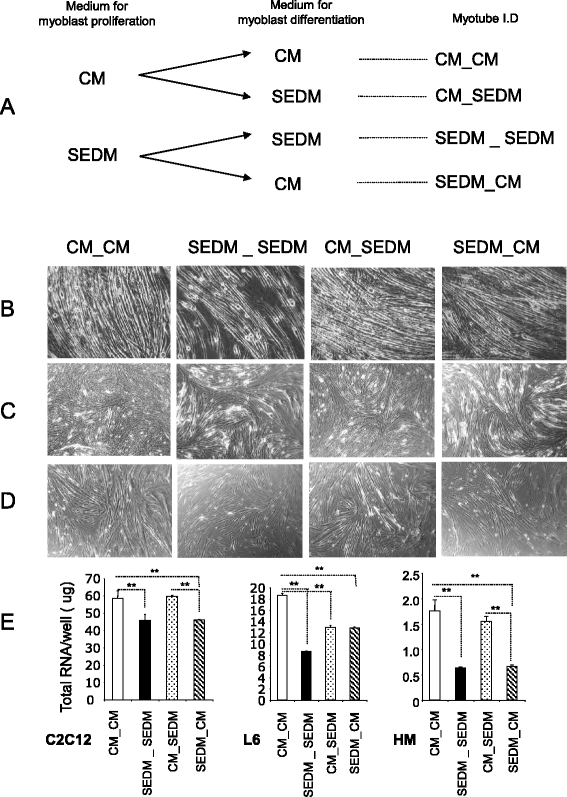
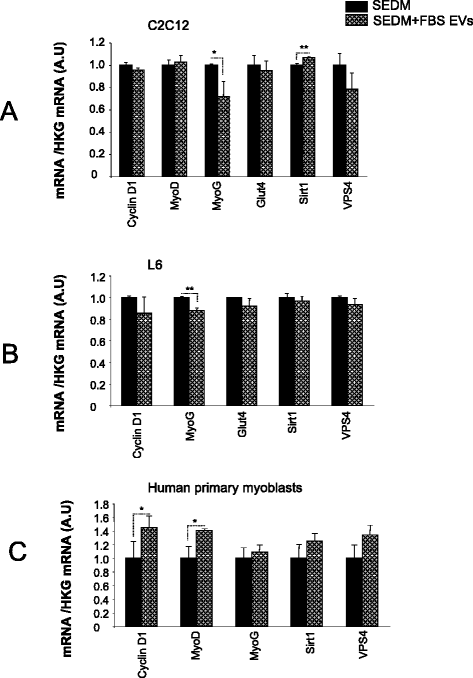
Results: Growth of myoblasts in EV-free media during proliferation produces the most unfavorable situation for proper myotube formation, when considering C212 and human myoblasts. Removing EVs from serum committed myoblasts to differentiate precociously (induction of myogenin and decreased expression of myomiR involved in myogenesis). C2C12 and human myoblasts, grown constantly in EV-depleted media during proliferation and differentiation, formed less myotubes than in CM. They had a reduced level of myogenin and a strong increase in myostatin expression, a negative regulator of muscle cell differentiation that affects myotube size. This situation was not reversed when confluent myoblasts were switched to CM for differentiation. Like C2C12 and human cells, L6 formed less myotubes in EVs-depleted media. However, as they do not express myostatin, L6 myotubes were larger and expressed higher level of CKTM2 compared to myotubes grown in CM suggesting that they had reached a higher level of differentiation.
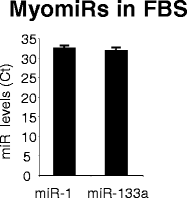
Conclusions: Researchers studying the role of muscle EVs in culture conditions should consider that depleting EVs from serum alters the phenotype of muscle cells. Interestingly, the cross-talk between myoblasts and myotubes during myogenesis (Forterre 2014, PLoS One. 2014 Jan 2;9(1):e84153) can be recapitulate by using FBS-EVs as well. This implies that EVs can transfer specific signals to cells from unrelated species and that part of serum EV composition is evolutionarily conserved (e.g.; myomiR are detected in FBS-EVs). EVs in body fluids could have an unsuspected function during embryogenesis and in regulation of cellular processes such as hypertrophy and hyperplasia.
Keywords: Bovine serum; C2C12; Extracellular vesicles; Gene expression; Human myoblasts; L6; Muscle cell; Proliferation; miRNAs.
Figures




References
-
- Kirikae T, Tamura H, Hashizume M, Kirikae F, Uemura Y, Tanaka S, Yokochi T, Nakano M. Endotoxin contamination in fetal bovine serum and its influence on tumor necrosis factor production by macrophage-like cells J774.1 cultured in the presence of the serum. Int J Immunopharmacol. 1997;19:255–62. doi: 10.1016/S0192-0561(97)00066-0. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

