Switching patients with acromegaly from octreotide to pasireotide improves biochemical control: crossover extension to a randomized, double-blind, Phase III study
- PMID: 27039081
- PMCID: PMC4818908
- DOI: 10.1186/s12902-016-0096-8
Switching patients with acromegaly from octreotide to pasireotide improves biochemical control: crossover extension to a randomized, double-blind, Phase III study
Abstract
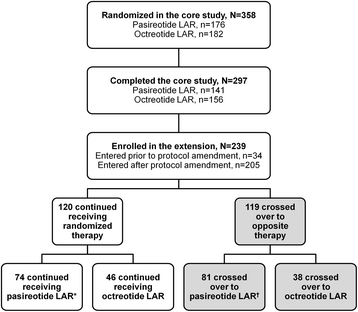
Background: Many patients with acromegaly do not achieve biochemical control with first-generation somatostatin analogues. A large, multicenter, randomized, Phase III core study demonstrated that pasireotide LAR had significantly superior efficacy over octreotide LAR. This analysis explores the efficacy and safety of switching therapeutic arms in inadequately controlled patients during a 12-month crossover extension.
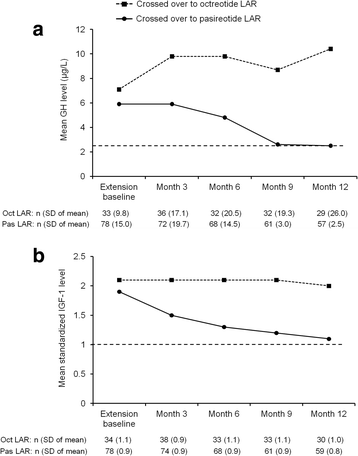
Methods: Patients with inadequate biochemical control (GH ≥2.5 μg/L and/or IGF-1 > ULN) at end of core study (month 12) were eligible to switch to pasireotide LAR 40 mg/28 days (n = 81) or octreotide LAR 20 mg/28 days (n = 38). One dose escalation to pasireotide LAR 60 mg/28 days or octreotide LAR 30 mg/28 days was permitted, but not mandatory, at month 17 or 20.
Results: Twelve months after crossover, 17.3 % of pasireotide LAR and 0 % of octreotide LAR patients achieved GH <2.5 μg/L and normal IGF-1 (main outcome measure); 27.2 and 5.3 % of pasireotide LAR and octreotide LAR patients achieved normal IGF-1, respectively; 44.4 and 23.7 % of pasireotide LAR and octreotide LAR patients achieved GH <2.5 μg/L, respectively. Mean (±SD) tumor volume further decreased from the end of the core study by 25 % (±25) and 18 % (±28); 54.3 % of pasireotide LAR and 42.3 % of octreotide LAR patients achieved significant (≥20 %) tumor volume reduction during the extension. The safety profile of pasireotide LAR was similar to that of octreotide LAR, with the exception of the frequency and degree of hyperglycemia-related adverse events.
Conclusions: Pasireotide LAR is a promising treatment option for patients with acromegaly inadequately controlled with the first-generation somatostatin analogue octreotide LAR.
Trial registration: clinicaltrials.gov, NCT00600886 . Registered 14 January 2008.
Keywords: Acromegaly; Crossover; Extension; Octreotide; Pasireotide.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

