Vitamin D immunoregulation through dendritic cells
- PMID: 27040466
- PMCID: PMC4913286
- DOI: 10.1111/imm.12610
Vitamin D immunoregulation through dendritic cells
Abstract
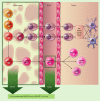
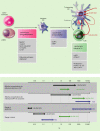
Vitamin D (VD3) has been linked to immunological processes, and its supplementation may have a role in treatment or prevention of diseases with underlying autoimmune or pro-inflammatory states. As initiators of the immune responses, dendritic cells (DC) are a potential target of VD3 to dampen autoimmunity and inflammation, but the role of DC in VD3-mediated immunomodulation in vivo is not understood. In addition to being targets of VD3, DC can provide a local source of bioactive VD3 for regulation of T-cell responses. Here we review existing studies that describe the tolerogenic potential of VD3 on DC, and discuss them in the context of current understanding of DC development and function. We speculate on mechanisms that might account for the potent but poorly understood tolerogenic activities of VD3 and the role of DC as both targets and sources of this hormone.
Keywords: dendritic cells; inflammation; vitamin D.
© 2016 The Authors. Immunology published by John Wiley & Sons Ltd.
Figures
Similar articles
-
Induction of Treg by monocyte-derived DC modulated by vitamin D3 or dexamethasone: differential role for PD-L1.Eur J Immunol. 2009 Nov;39(11):3147-59. doi: 10.1002/eji.200839103. Eur J Immunol. 2009. PMID: 19688742
-
Dendritic cells modified by vitamin D: future immunotherapy for autoimmune diseases.Vitam Horm. 2011;86:63-82. doi: 10.1016/B978-0-12-386960-9.00003-4. Vitam Horm. 2011. PMID: 21419267 Review.
-
Delivery of alloantigens via apoptotic cells generates dendritic cells with an immature tolerogenic phenotype.Transplant Proc. 2011 Jul-Aug;43(6):2325-33. doi: 10.1016/j.transproceed.2011.06.007. Transplant Proc. 2011. PMID: 21839264
-
Modulation of the dendritic cell-T-cell synapse to promote pathogen immunity and prevent autoimmunity.Immunotherapy. 2011 Apr;3(4 Suppl):6-11. doi: 10.2217/imt.11.38. Immunotherapy. 2011. PMID: 21524159 Review.
-
Evolving Role of Vitamin D in Immune-Mediated Disease and Its Implications in Autoimmune Hepatitis.Dig Dis Sci. 2019 Feb;64(2):324-344. doi: 10.1007/s10620-018-5351-6. Epub 2018 Oct 28. Dig Dis Sci. 2019. PMID: 30370494 Review.
Cited by
-
Micronutrients as immunomodulatory tools for COVID-19 management.Clin Immunol. 2020 Nov;220:108545. doi: 10.1016/j.clim.2020.108545. Epub 2020 Jul 22. Clin Immunol. 2020. PMID: 32710937 Free PMC article. Review.
-
Decreased preoperative serum 25-Hydroxyvitamin D levels in colorectal cancer are associated with systemic inflammation and serrated morphology.Sci Rep. 2016 Nov 7;6:36519. doi: 10.1038/srep36519. Sci Rep. 2016. PMID: 27819306 Free PMC article.
-
The Role of Vitamins in the Pathogenesis of Asthma.Int J Mol Sci. 2023 May 10;24(10):8574. doi: 10.3390/ijms24108574. Int J Mol Sci. 2023. PMID: 37239921 Free PMC article. Review.
-
Impact of Vitamin D on Immunopathology of Hashimoto's Thyroiditis: From Theory to Practice.Nutrients. 2023 Jul 17;15(14):3174. doi: 10.3390/nu15143174. Nutrients. 2023. PMID: 37513592 Free PMC article. Review.
-
Cross-sectional associations between vitamin D status and periodontitis in adults in Northern Norway: The Tromsø Study 2015-2016.BMC Oral Health. 2025 May 30;25(1):856. doi: 10.1186/s12903-025-06261-2. BMC Oral Health. 2025. PMID: 40448051 Free PMC article.
References
-
- Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol 2013; 31:563–604. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3853342&tool=p.... - PMC - PubMed
-
- Kiss M, Czimmerer Z, Nagy L. The role of lipid‐activated nuclear receptors in shaping macrophage and dendritic cell function: from physiology to pathology. J Allergy Clin Immunol 2013; 132:264–86. URL http://dx.doi.org/10.1016/j.jaci.2013.05.044. - DOI - PubMed
-
- Veldhoen M, Brucklacher‐Waldert V. Dietary influences on intestinal immunity. Nat Rev Immunol 2012; 12:696–708. URL http://www.ncbi.nlm.nih.gov/pubmed/23007570. - PubMed
-
- Mora JR, Iwata M, von Andrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol 2008; 8:685–98. URL http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2906676&tool=p.... - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

