Mechanisms by which Stress Affects the Experimental and Clinical Inflammatory Bowel Disease (IBD): Role of Brain-Gut Axis
- PMID: 27040468
- PMCID: PMC5333596
- DOI: 10.2174/1570159x14666160404124127
Mechanisms by which Stress Affects the Experimental and Clinical Inflammatory Bowel Disease (IBD): Role of Brain-Gut Axis
Abstract
Background: Stress of different origin is known to alter so called "braingut axis" and contributes to a broad array of gastrointestinal disorders including inflammatory bowel disease (IBD), irritable bowel syndrome (IBS) and other functional gastrointestinal diseases. The stressful situations and various stressors including psychosocial events, heat, hypo- and hyperthermia may worsen the course of IBD via unknown mechanism. The aims of this paper were to provide an overview of experimental and clinical evidences that stress activates the brain-gut axis which results in a mucosal mast cells activation and an increase in the production of proinflammatory cytokines and other endocrine and humoral mediators.
Methods: Research and online content related to effects of stress on lower bowel disorders are reviewed and most important mechanisms are delineated.
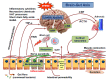
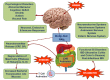
Results: Brain conveys the neural, endocrine and circulatory messages to the gut via brain-gut axis reflecting changes in corticotrophin releasing hormone, mast cells activity, neurotransmission at the autonomic nerves system and intestinal barrier function all affecting the pathogenesis of animal colitis and human IBD. Stress triggers the hypothalamus-pituitary axis and the activation of the autonomic nervous system, an increase in cortisol levels and proinflammatory cytokines such as tumor necrosis factor-alpha, interleukin-8, interleukin-1beta and interleukin-6.
Conclusion: The acute or chronic stress enhances the intestinal permeability weakening of the tight junctions and increasing bacterial translocation into the intestinal wall. An increased microbial load in the colonic tissue, excessive cytokine release and a partially blunted immune reactivity in response to stress result in its negative impact on IBD.
Figures
References
-
- Oliver S.R., Phillips N.A., Novosad V.L., Bakos M.P., Talbert E.E., Clanton T.L. Hyperthermia induces injury to the intestinal mucosa in the mouse: evidence for an oxidative stress mechanism. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012;302(7):R845–R853. [http://dx.doi.org/10.1152/ajpregu.00595.2011]. [PMID: 22237593]. - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
