The Antagonistic Gene Paralogs Upf3a and Upf3b Govern Nonsense-Mediated RNA Decay
- PMID: 27040500
- PMCID: PMC4826573
- DOI: 10.1016/j.cell.2016.02.046
The Antagonistic Gene Paralogs Upf3a and Upf3b Govern Nonsense-Mediated RNA Decay
Abstract
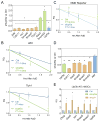
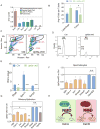
Gene duplication is a major evolutionary force driving adaptation and speciation, as it allows for the acquisition of new functions and can augment or diversify existing functions. Here, we report a gene duplication event that yielded another outcome--the generation of antagonistic functions. One product of this duplication event--UPF3B--is critical for the nonsense-mediated RNA decay (NMD) pathway, while its autosomal counterpart--UPF3A--encodes an enigmatic protein previously shown to have trace NMD activity. Using loss-of-function approaches in vitro and in vivo, we discovered that UPF3A acts primarily as a potent NMD inhibitor that stabilizes hundreds of transcripts. Evidence suggests that UPF3A acquired repressor activity through simple impairment of a critical domain, a rapid mechanism that may have been widely used in evolution. Mice conditionally lacking UPF3A exhibit "hyper" NMD and display defects in embryogenesis and gametogenesis. Our results support a model in which UPF3A serves as a molecular rheostat that directs developmental events.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures





Comment in
-
UPF3 Paralogs Wrestle for Fertility Influence.Biol Reprod. 2016 Jun;94(6):135. doi: 10.1095/biolreprod.116.141887. Epub 2016 May 18. Biol Reprod. 2016. PMID: 27339935 No abstract available.
Similar articles
-
Human UPF3A and UPF3B enable fault-tolerant activation of nonsense-mediated mRNA decay.EMBO J. 2022 May 16;41(10):e109191. doi: 10.15252/embj.2021109191. Epub 2022 Apr 22. EMBO J. 2022. PMID: 35451084 Free PMC article.
-
Mammalian UPF3A and UPF3B can activate nonsense-mediated mRNA decay independently of their exon junction complex binding.EMBO J. 2022 May 16;41(10):e109202. doi: 10.15252/embj.2021109202. Epub 2022 Apr 22. EMBO J. 2022. PMID: 35451102 Free PMC article.
-
UPF3A is dispensable for nonsense-mediated mRNA decay in mouse pluripotent and somatic cells.Life Sci Alliance. 2023 Mar 30;6(6):e202201589. doi: 10.26508/lsa.202201589. Print 2023 Jun. Life Sci Alliance. 2023. PMID: 36997282 Free PMC article.
-
RNA decay, evolution, and the testis.RNA Biol. 2017 Feb;14(2):146-155. doi: 10.1080/15476286.2016.1265199. Epub 2016 Dec 2. RNA Biol. 2017. PMID: 27911186 Free PMC article. Review.
-
Functional roles of human Up-frameshift suppressor 3 (UPF3) proteins: From nonsense-mediated mRNA decay to neurodevelopmental disorders.Biochimie. 2021 Jan;180:10-22. doi: 10.1016/j.biochi.2020.10.011. Epub 2020 Oct 24. Biochimie. 2021. PMID: 33132159 Review.
Cited by
-
Identification of germ cell-specific Mga variant mRNA that promotes meiosis via impediment of a non-canonical PRC1.Sci Rep. 2021 May 6;11(1):9737. doi: 10.1038/s41598-021-89123-5. Sci Rep. 2021. PMID: 33958653 Free PMC article.
-
Regulation of nonsense-mediated mRNA decay in neural development and disease.J Mol Cell Biol. 2021 Aug 4;13(4):269-281. doi: 10.1093/jmcb/mjab022. J Mol Cell Biol. 2021. PMID: 33783512 Free PMC article. Review.
-
Some ASOs that bind in the coding region of mRNAs and induce RNase H1 cleavage can cause increases in the pre-mRNAs that may blunt total activity.Nucleic Acids Res. 2020 Sep 25;48(17):9840-9858. doi: 10.1093/nar/gkaa715. Nucleic Acids Res. 2020. PMID: 32870273 Free PMC article.
-
Nonsense-mediated mRNA decay: a 'nonsense' pathway makes sense in stem cell biology.Nucleic Acids Res. 2018 Feb 16;46(3):1038-1051. doi: 10.1093/nar/gkx1272. Nucleic Acids Res. 2018. PMID: 29272451 Free PMC article. Review.
-
Nonsense-Mediated mRNA Decay: Mechanistic Insights and Physiological Significance.Mol Biotechnol. 2024 Nov;66(11):3077-3091. doi: 10.1007/s12033-023-00927-4. Epub 2023 Nov 6. Mol Biotechnol. 2024. PMID: 37930508 Review.
References
-
- Bastos H, Lassalle B, Chicheportiche A, Riou L, Testart J, Allemand I, Fouchet P. Flow cytometric characterization of viable meiotic and postmeiotic cells by Hoechst 33342 in mouse spermatogenesis 2005 - PubMed
-
- Boelz S, Neu-Yilik G, Gehring NH, Hentze MW, Kulozik AE. A chemiluminescence-based reporter system to monitor nonsense-mediated mRNA decay 2006 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

