Dcc Mediates Functional Assembly of Peripheral Auditory Circuits
- PMID: 27040640
- PMCID: PMC4819185
- DOI: 10.1038/srep23799
Dcc Mediates Functional Assembly of Peripheral Auditory Circuits
Abstract
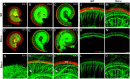
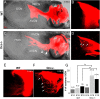
Proper structural organization of spiral ganglion (SG) innervation is crucial for normal hearing function. However, molecular mechanisms underlying the developmental formation of this precise organization remain not well understood. Here, we report in the developing mouse cochlea that deleted in colorectal cancer (Dcc) contributes to the proper organization of spiral ganglion neurons (SGNs) within the Rosenthal's canal and of SGN projections toward both the peripheral and central auditory targets. In Dcc mutant embryos, mispositioning of SGNs occurred along the peripheral auditory pathway with misrouted afferent fibers and reduced synaptic contacts with hair cells. The central auditory pathway simultaneously exhibited similar defective phenotypes as in the periphery with abnormal exit of SGNs from the Rosenthal's canal towards central nuclei. Furthermore, the axons of SGNs ascending into the cochlear nucleus had disrupted bifurcation patterns. Thus, Dcc is necessary for establishing the proper spatial organization of SGNs and their fibers in both peripheral and central auditory pathways, through controlling axon targeting and cell migration. Our results suggest that Dcc plays an important role in the developmental formation of peripheral and central auditory circuits, and its mutation may contribute to sensorineural hearing loss.
Figures






Similar articles
-
EphA7 regulates spiral ganglion innervation of cochlear hair cells.Dev Neurobiol. 2016 Apr;76(4):452-69. doi: 10.1002/dneu.22326. Epub 2015 Jul 27. Dev Neurobiol. 2016. PMID: 26178595 Free PMC article.
-
Mutation of Npr2 leads to blurred tonotopic organization of central auditory circuits in mice.PLoS Genet. 2014 Dec 4;10(12):e1004823. doi: 10.1371/journal.pgen.1004823. eCollection 2014 Dec. PLoS Genet. 2014. PMID: 25473838 Free PMC article.
-
Slit/Robo signaling mediates spatial positioning of spiral ganglion neurons during development of cochlear innervation.J Neurosci. 2013 Jul 24;33(30):12242-54. doi: 10.1523/JNEUROSCI.5736-12.2013. J Neurosci. 2013. PMID: 23884932 Free PMC article.
-
Recent advances in the development and function of type II spiral ganglion neurons in the mammalian inner ear.Semin Cell Dev Biol. 2017 May;65:80-87. doi: 10.1016/j.semcdb.2016.09.017. Epub 2016 Oct 17. Semin Cell Dev Biol. 2017. PMID: 27760385 Free PMC article. Review.
-
Talking back: Development of the olivocochlear efferent system.Wiley Interdiscip Rev Dev Biol. 2018 Nov;7(6):e324. doi: 10.1002/wdev.324. Epub 2018 Jun 26. Wiley Interdiscip Rev Dev Biol. 2018. PMID: 29944783 Free PMC article. Review.
Cited by
-
Molecular logic for cellular specializations that initiate the auditory parallel processing pathways.bioRxiv [Preprint]. 2024 Oct 6:2023.05.15.539065. doi: 10.1101/2023.05.15.539065. bioRxiv. 2024. Update in: Nat Commun. 2025 Jan 9;16(1):489. doi: 10.1038/s41467-024-55257-z. PMID: 37293040 Free PMC article. Updated. Preprint.
-
Wiring the senses: Factors that regulate peripheral axon pathfinding in sensory systems.Dev Dyn. 2023 Jan;252(1):81-103. doi: 10.1002/dvdy.523. Epub 2022 Aug 30. Dev Dyn. 2023. PMID: 35972036 Free PMC article. Review.
-
Netrin-1 Confines Rhombic Lip-Derived Neurons to the CNS.Cell Rep. 2018 Feb 13;22(7):1666-1680. doi: 10.1016/j.celrep.2018.01.068. Cell Rep. 2018. PMID: 29444422 Free PMC article.
-
Reviving-like prosocial behavior in response to unconscious or dead conspecifics in rodents.Science. 2025 Feb 21;387(6736):eadq2677. doi: 10.1126/science.adq2677. Epub 2025 Feb 21. Science. 2025. PMID: 39977514 Free PMC article.
-
ISL1 is necessary for auditory neuron development and contributes toward tonotopic organization.Proc Natl Acad Sci U S A. 2022 Sep 13;119(37):e2207433119. doi: 10.1073/pnas.2207433119. Epub 2022 Sep 8. Proc Natl Acad Sci U S A. 2022. PMID: 36074819 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

