Effect of the achondroplasia mutation on FGFR3 dimerization and FGFR3 structural response to fgf1 and fgf2: A quantitative FRET study in osmotically derived plasma membrane vesicles
- PMID: 27040652
- PMCID: PMC4870120
- DOI: 10.1016/j.bbamem.2016.03.027
Effect of the achondroplasia mutation on FGFR3 dimerization and FGFR3 structural response to fgf1 and fgf2: A quantitative FRET study in osmotically derived plasma membrane vesicles
Abstract
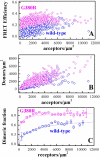
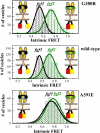
The G380R mutation in the transmembrane domain of FGFR3 is a germline mutation responsible for most cases of Achondroplasia, a common form of human dwarfism. Here we use quantitative Fӧster Resonance Energy Transfer (FRET) and osmotically derived plasma membrane vesicles to study the effect of the achondroplasia mutation on the early stages of FGFR3 signaling in response to the ligands fgf1 and fgf2. Using a methodology that allows us to capture structural changes on the cytoplasmic side of the membrane in response to ligand binding to the extracellular domain of FGFR3, we observe no measurable effects of the G380R mutation on FGFR3 ligand-bound dimer configurations. Instead, the most notable effect of the achondroplasia mutation is increased propensity for FGFR3 dimerization in the absence of ligand. This work reveals new information about the molecular events that underlie the achondroplasia phenotype, and highlights differences in FGFR3 activation due to different single amino-acid pathogenic mutations.
Keywords: Achondroplasia; Dimer stability; Dimerization; Fibroblast growth factor receptor 3; Receptor tyrosine kinases; skeletal disorders.
Copyright © 2016 Elsevier B.V. All rights reserved.
Figures


References
-
- Horton WA, Hall JG, Hecht JT. Achondroplasia. Lancet. 2007;370:162–172. - PubMed
-
- Shiang R, Thompson LM, Zhu Y-Z, Church DM, Fielder TJ, Bocian M, Winokur ST, Wasmuth JJ. Mutations in the transmembrane domain of FGFR3 cause the most common genetic form of dwarfism, achondroplasia. Cell. 1994;78:335–342. - PubMed
-
- Deng C, Wynshaw-Boris A, Zhou F, Kuo A, Leder P. Fibroblast growth factor receptor 3 is a negative regulator of bone growth. Cell. 1996;84:911–921. - PubMed
-
- Cohen MM. Some chondrodysplasias with short limbs: molecular perspectives. Am. J. Med. Genet. 2002;112:304–313. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

