Inhibition, Not Excitation, Drives Rhythmic Whisking
- PMID: 27041498
- PMCID: PMC4929009
- DOI: 10.1016/j.neuron.2016.03.007
Inhibition, Not Excitation, Drives Rhythmic Whisking
Abstract
Sniffing and whisking typify the exploratory behavior of rodents. These actions involve separate oscillators in the medulla, located respectively in the pre-Bötzinger complex (preBötC) and the vibrissa-related region of the intermediate reticular formation (vIRt). We examine how these oscillators synergize to control sniffing and whisking. We find that the vIRt contains glycinergic/GABAergic cells that rhythmically inhibit vibrissa facial motoneurons. As a basis for the entrainment of whisking by breathing, but not vice versa, we provide evidence for unidirectional connections from the preBötC to the vIRt. The preBötC further contributes to the control of the mystacial pad. Lastly, we show that bilateral synchrony of whisking relies on the respiratory rhythm, consistent with commissural connections between preBötC cells. These data yield a putative circuit in which the preBötC acts as a master clock for the synchronization of vibrissa, pad, and snout movements, as well as for the bilateral synchronization of whisking.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures
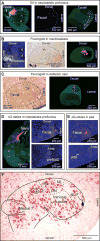


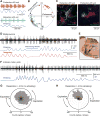


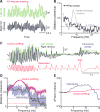

Comment in
-
Inhibition Patterns the Whisking Rhythm.Neuron. 2016 Apr 20;90(2):211-3. doi: 10.1016/j.neuron.2016.04.012. Neuron. 2016. PMID: 27100193
References
-
- Bouvier J, Thoby-Brisson M, Renier N, Dubreuil V, Ericson J, Champagnat J, Pierani A, Chédotal A, Fortin G. Hindbrain interneurons and axon guidance signaling critical for breathing. Nat Neurosci. 2010;3:1066–1074. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

