Divergent Routing of Positive and Negative Information from the Amygdala during Memory Retrieval
- PMID: 27041499
- PMCID: PMC4854303
- DOI: 10.1016/j.neuron.2016.03.004
Divergent Routing of Positive and Negative Information from the Amygdala during Memory Retrieval
Abstract
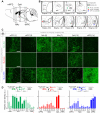
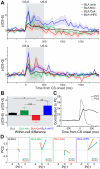
Although the basolateral amygdala (BLA) is known to play a critical role in the formation of memories of both positive and negative valence, the coding and routing of valence-related information is poorly understood. Here, we recorded BLA neurons during the retrieval of associative memories and used optogenetic-mediated phototagging to identify populations of neurons that synapse in the nucleus accumbens (NAc), the central amygdala (CeA), or ventral hippocampus (vHPC). We found that despite heterogeneous neural responses within each population, the proportions of BLA-NAc neurons excited by reward predictive cues and of BLA-CeA neurons excited by aversion predictive cues were higher than within the entire BLA. Although the BLA-vHPC projection is known to drive behaviors of innate negative valence, these neurons did not preferentially code for learned negative valence. Together, these findings suggest that valence encoding in the BLA is at least partially mediated via divergent activity of anatomically defined neural populations.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures





Comment in
-
Parsing Reward and Aversion in the Amygdala.Neuron. 2016 Apr 20;90(2):209-11. doi: 10.1016/j.neuron.2016.04.011. Neuron. 2016. PMID: 27100192
References
-
- Ben-Ari Y, Tremblay E, Ottersen OP. Injections of kainic acid into the amygdaloid complex of the rat: an electrographic, clinical and histological study in relation to the pathology of epilepsy. Neuroscience. 1980;5:515–528. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
