Modulation of liver tolerance by conventional and nonconventional antigen-presenting cells and regulatory immune cells
- PMID: 27041638
- PMCID: PMC4856800
- DOI: 10.1038/cmi.2015.112
Modulation of liver tolerance by conventional and nonconventional antigen-presenting cells and regulatory immune cells
Abstract
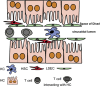
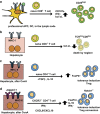
The liver is a tolerogenic organ with exquisite mechanisms of immune regulation that ensure upkeep of local and systemic immune tolerance to self and foreign antigens, but that is also able to mount effective immune responses against pathogens. The immune privilege of liver allografts was recognized first in pigs in spite of major histo-compatibility complex mismatch, and termed the "liver tolerance effect". Furthermore, liver transplants are spontaneously accepted with only low-dose immunosuppression, and induce tolerance for non-hepatic co-transplanted allografts of the same donor. Although this immunotolerogenic environment is favorable in the setting of organ transplantation, it is detrimental in chronic infectious liver diseases like hepatitis B or C, malaria, schistosomiasis or tumorigenesis, leading to pathogen persistence and weak anti-tumor effects. The liver is a primary site of T-cell activation, but it elicits poor or incomplete activation of T cells, leading to their abortive activation, exhaustion, suppression of their effector function and early death. This is exploited by pathogens and can impair pathogen control and clearance or allow tumor growth. Hepatic priming of T cells is mediated by a number of local conventional and nonconventional antigen-presenting cells (APCs), which promote tolerance by immune deviation, induction of T-cell anergy or apoptosis, and generating and expanding regulatory T cells. This review will focus on the communication between classical and nonclassical APCs and lymphocytes in the liver in tolerance induction and will discuss recent insights into the role of innate lymphocytes in this process.
Figures


References
-
- Tiegs G, Lohse AW. Immune tolerance: what is unique about the liver. J Autoimmun 2010; 34: 1–6. - PubMed
-
- Thomson AW, Knolle PA. Antigen-presenting cell function in the tolerogenic liver environment. Nat Rev Immunol 2010; 10: 753–766. - PubMed
-
- Gao B, Jeong WI, Tian Z. Liver: an organ with predominant innate immunity. Hepatology 2008; 47: 729–736. - PubMed
-
- Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol 2009; 27: 147–163. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

