Regional differences in hepatitis C treatment with peginterferon and ribavirin in Japan: a retrospective cohort study
- PMID: 27042013
- PMCID: PMC4809336
- DOI: 10.2147/DDDT.S102458
Regional differences in hepatitis C treatment with peginterferon and ribavirin in Japan: a retrospective cohort study
Abstract
Purpose: The aims of this study were to investigate regional differences in hepatitis C virus (HCV) infection treatment with peginterferon and ribavirin in Japan and to develop and validate statistical models for analysis of regional differences, using generalized linear mixed models.
Methods: Individuals with chronic HCV infection were identified from the Japanese Interferon Database (registered from December 2009 to April 2013). The total sustained virologic response rate and the rate in each prefecture were calculated. In the analysis using generalized linear mixed models, the following four models were constructed: 1) prefecture as a fixed effect, 2) prefecture and other confounding variables as fixed effects, 3) prefecture as a random effect, and 4) prefecture as a random effect and other confounding variables as fixed effects. The quality of the model fit was assessed using the Akaike information criterion and the Bayesian information criterion. All statistical analyses were performed using SAS Version 9.4 for Windows.
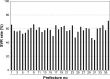
Results: From 36 prefectures, 16,349 cases were recorded in the study period. Of these, 4,677 were excluded according to certain criteria. The total sustained virologic response rate was 59.9% (range, 43.9%-71.6%). The statistical model including prefecture as a random effect and other confounding variables as fixed effects showed the best fit based on the Akaike information criterion (13,830.92) and Bayesian information criterion (13,845.17).
Conclusion: Regional differences may exist in HCV infection treatment in Japan. The model including prefecture as a random effect and other confounding variables as fixed effects was appropriate for analysis of such regional differences. Additional studies considering the medical situations of each patient would provide useful information that could contribute to improve and standardize HCV infection treatment.
Keywords: generalized linear mixed model; hepatitis C virus; interferon; nationwide database; regional differences.
Figures

Similar articles
-
Regional Differences in Hepatitis C Treatment with Peginterferon and Ribavirin in Japan in Both Genotype 1 and Genotype 2: A Retrospective Cohort Study.Biol Pharm Bull. 2016 Sep 1;39(9):1538-43. doi: 10.1248/bpb.b16-00408. Epub 2016 Jul 7. Biol Pharm Bull. 2016. PMID: 27384444
-
Peginterferon alfa-2a plus ribavirin for the treatment of dual chronic infection with hepatitis B and C viruses.Gastroenterology. 2009 Feb;136(2):496-504.e3. doi: 10.1053/j.gastro.2008.10.049. Epub 2008 Oct 29. Gastroenterology. 2009. PMID: 19084016 Clinical Trial.
-
Consensus Interferon for Recurrent Hepatitis C Infection in Nonresponders to Peginterferon and Ribavirin After Liver Transplant.Exp Clin Transplant. 2015 Dec;13(6):543-9. Exp Clin Transplant. 2015. PMID: 26643674
-
A comparison of peginterferon α-2a and α-2b for treatment-naive patients with chronic hepatitis C virus: A meta-analysis of randomized trials.Clin Ther. 2010 Aug;32(9):1565-77. doi: 10.1016/j.clinthera.2010.08.009. Clin Ther. 2010. PMID: 20974315 Review.
-
Management of hepatitis C.Br Med Bull. 2004 Oct 4;70:51-69. doi: 10.1093/bmb/ldh022. Print 2004. Br Med Bull. 2004. PMID: 15466491 Review.
References
-
- World Health Organization [webpage on the Internet] Hepatitis C Fact Sheet No. 164 July 2015. 2015. [Accessed August 12, 2015]. Available from: http://www.who.int/mediacentre/factsheets/fs164/en/
-
- Averhoff FM, Glass N, Holtzman D. Global burden of hepatitis C: considerations for healthcare providers in the United States. Clin Infect Dis. 2012;55(suppl 1):S10–S15. - PubMed
-
- Ghany MG, Nelson DR, Strader DB, Thomas DL, Seeff LB, American Association for Study of Liver Diseases An update on treatment of genotype 1 chronic hepatitis C virus infection: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011;54:1433–1444. - PMC - PubMed
-
- Gane EJ, Stedman CA, Hyland RH, et al. Nucleotide polymerase inhibitor sofosbuvir plus ribavirin for hepatitis C. N Engl J Med. 2013;368:34–44. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

