Dimethyl fumarate in the management of multiple sclerosis: appropriate patient selection and special considerations
- PMID: 27042079
- PMCID: PMC4780395
- DOI: 10.2147/TCRM.S85099
Dimethyl fumarate in the management of multiple sclerosis: appropriate patient selection and special considerations
Abstract
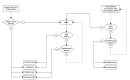
Delayed-release dimethyl fumarate (DMF), also known as gastroresistant DMF, is the most recently approved oral disease-modifying treatment (DMT) for relapsing multiple sclerosis. Two randomized clinical trials (Determination of the Efficacy and Safety of Oral Fumarate in Relapsing-Remitting MS [DEFINE] and Comparator and an Oral Fumarate in Relapsing-Remitting Multiple Sclerosis [CONFIRM]) demonstrated significant efficacy in reducing relapse rate and radiological signs of disease activity, as seen on magnetic resonance imaging. The DEFINE study also indicated a significant effect of DMF on disability worsening, while the low incidence of confirmed disability worsening in the CONFIRM trial rendered an insignificant reduction among the DMF-treated groups when compared to placebo. DMF also demonstrated a good safety profile and acceptable tolerability, since the most common side effects (gastrointestinal events and flushing reactions) are usually transient and mild to moderate in severity. Here, we discuss the place in therapy of DMF for individuals with relapsing multiple sclerosis, providing a tentative therapeutic algorithm to manage newly diagnosed patients and those who do not adequately respond to self-injectable DMTs. Literature data supporting the potential role of DMF as a first-line therapy are presented. The possibility of using DMF as switching treatment or even as an add-on strategy in patients with breakthrough disease despite self-injectable DMTs will also be discussed. Lastly, we argue about the role of DMF as an exit strategy from natalizumab-treated patients who are considered at risk for developing multifocal progressive leukoencephalopathy.
Keywords: dimethyl fumarate; multiple sclerosis; oral drugs; therapeutic algorithm.
Figures
Similar articles
-
Efficacy and Safety of Delayed-release Dimethyl Fumarate for Relapsing-remitting Multiple Sclerosis in Prior Interferon Users: An Integrated Analysis of DEFINE and CONFIRM.Clin Ther. 2017 Aug;39(8):1671-1679. doi: 10.1016/j.clinthera.2017.06.012. Epub 2017 Jul 25. Clin Ther. 2017. PMID: 28751099 Clinical Trial.
-
Comparative Efficacy and Safety of Ozanimod and Dimethyl Fumarate for Relapsing-Remitting Multiple Sclerosis Using Matching-Adjusted Indirect Comparison.CNS Drugs. 2021 Jul;35(7):795-804. doi: 10.1007/s40263-021-00805-0. Epub 2021 Apr 13. CNS Drugs. 2021. PMID: 33847901 Free PMC article.
-
Efficacy and Safety Outcomes with Diroximel Fumarate After Switching from Prior Therapies or Continuing on DRF: Results from the Phase 3 EVOLVE-MS-1 Study.Adv Ther. 2022 Apr;39(4):1810-1831. doi: 10.1007/s12325-022-02068-7. Epub 2022 Feb 24. Adv Ther. 2022. PMID: 35211872 Free PMC article. Clinical Trial.
-
Long-term safety and efficacy of dimethyl fumarate for up to 13 years in patients with relapsing-remitting multiple sclerosis: Final ENDORSE study results.Mult Scler. 2022 Apr;28(5):801-816. doi: 10.1177/13524585211037909. Epub 2021 Sep 1. Mult Scler. 2022. PMID: 34465252 Free PMC article. Clinical Trial.
-
[Dimethyl fumarate (tecfidera) is the first line treatment choice in patients with remitting multiple sclerosis].Zh Nevrol Psikhiatr Im S S Korsakova. 2017;117(11):140-145. doi: 10.17116/jnevro2017117111140-145. Zh Nevrol Psikhiatr Im S S Korsakova. 2017. PMID: 29265100 Review. Russian.
Cited by
-
Dimethyl fumarate decreases short-term but not long-term inflammation in a focal EAE model of neuroinflammation.EJNMMI Res. 2022 Feb 2;12(1):6. doi: 10.1186/s13550-022-00878-y. EJNMMI Res. 2022. PMID: 35107664 Free PMC article.
-
Nrf2 Signaling Pathway: a Potential Therapeutic Target in Combating Oxidative Stress and Neurotoxicity in Chemotherapy-Induced Cognitive Impairment.Mol Neurobiol. 2024 Feb;61(2):593-608. doi: 10.1007/s12035-023-03559-6. Epub 2023 Aug 29. Mol Neurobiol. 2024. PMID: 37644279 Review.
-
Precision Electrophile Tagging in Caenorhabditis elegans.Biochemistry. 2018 Jan 16;57(2):216-220. doi: 10.1021/acs.biochem.7b00642. Epub 2017 Sep 12. Biochemistry. 2018. PMID: 28857552 Free PMC article.
-
Recent advances in understanding NRF2 as a druggable target: development of pro-electrophilic and non-covalent NRF2 activators to overcome systemic side effects of electrophilic drugs like dimethyl fumarate.F1000Res. 2017 Dec 14;6:2138. doi: 10.12688/f1000research.12111.1. eCollection 2017. F1000Res. 2017. PMID: 29263788 Free PMC article. Review.
-
The Complex Genetic and Epigenetic Regulation of the Nrf2 Pathways: A Review.Antioxidants (Basel). 2023 Feb 3;12(2):366. doi: 10.3390/antiox12020366. Antioxidants (Basel). 2023. PMID: 36829925 Free PMC article. Review.
References
-
- Compston A, Coles A. Multiple sclerosis. Lancet. 2002;359(9313):1221–1231. - PubMed
-
- Brück W, Stadelmann C. The spectrum of multiple sclerosis: new lessons from pathology. Curr Opin Neurol. 2005;18(3):221–224. - PubMed
-
- Kutzelnigg A, Lassmann H. Pathology of multiple sclerosis and related inflammatory demyelinating diseases. Handb Clin Neurol. 2014;122:15–58. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

