Inhibition of fatty acid desaturation is detrimental to cancer cell survival in metabolically compromised environments
- PMID: 27042297
- PMCID: PMC4818530
- DOI: 10.1186/s40170-016-0146-8
Inhibition of fatty acid desaturation is detrimental to cancer cell survival in metabolically compromised environments
Abstract
Background: Enhanced macromolecule biosynthesis is integral to growth and proliferation of cancer cells. Lipid biosynthesis has been predicted to be an essential process in cancer cells. However, it is unclear which enzymes within this pathway offer the best selectivity for cancer cells and could be suitable therapeutic targets.
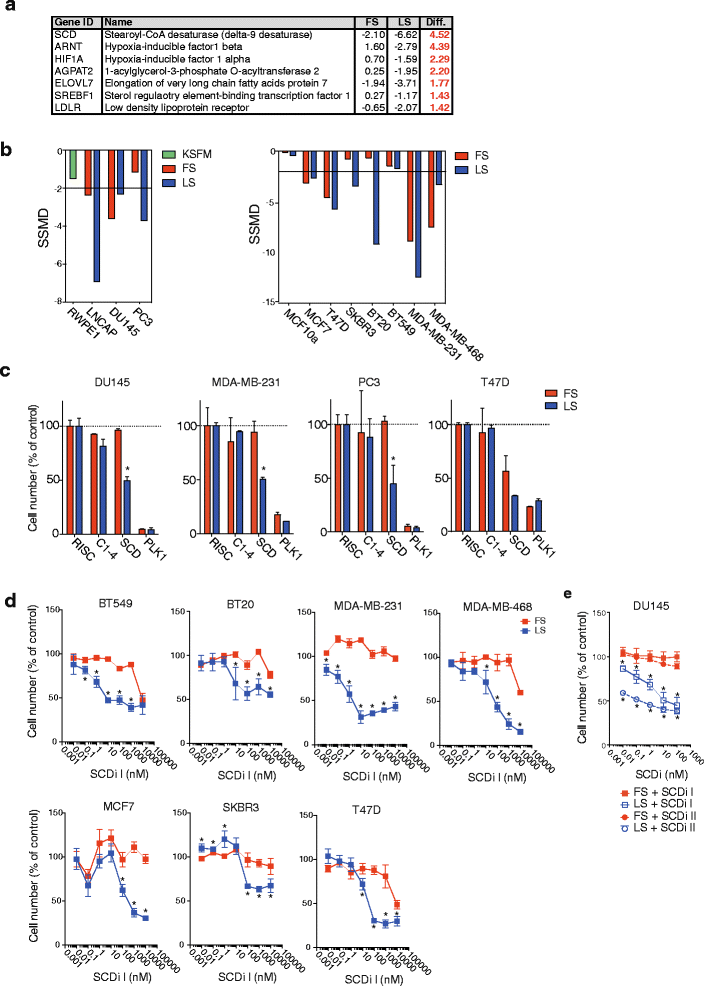
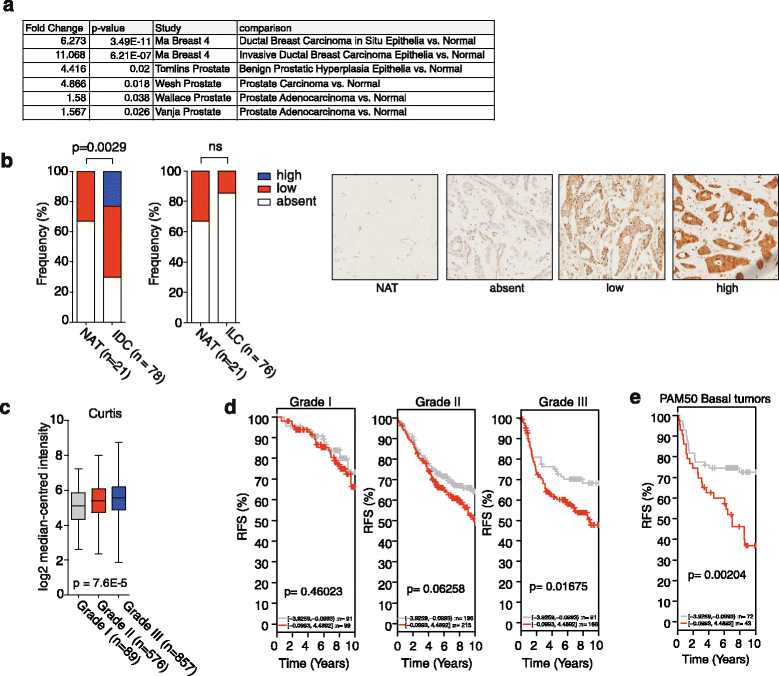
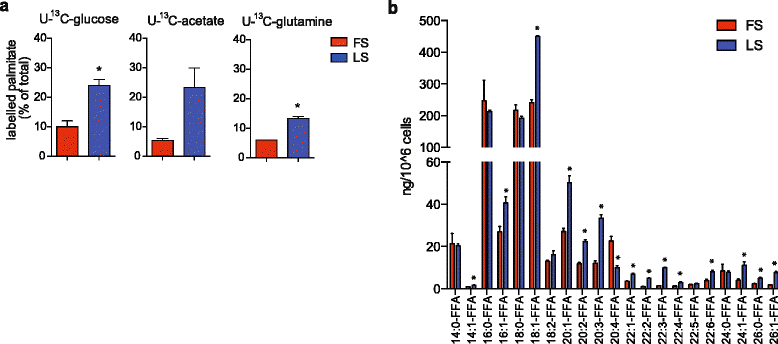
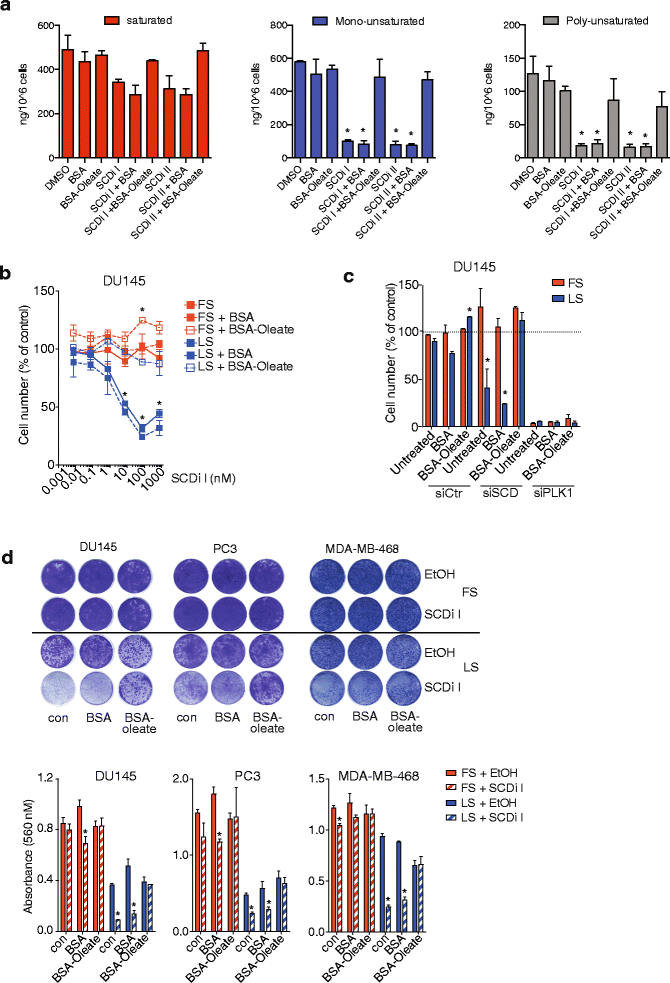
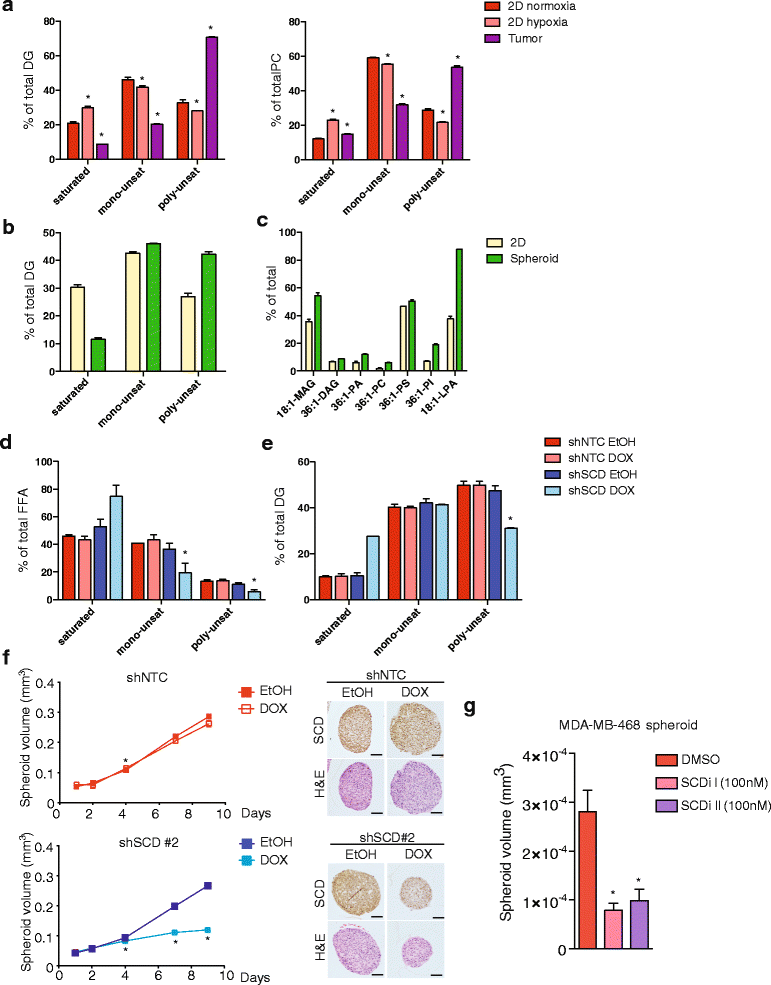
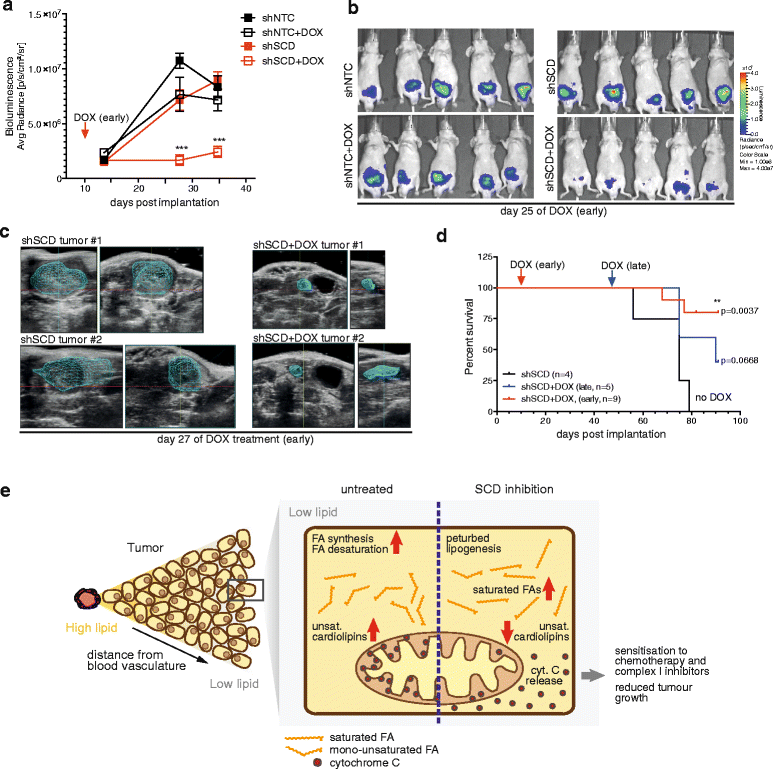
Results: Using functional genomics, we identified stearoyl-CoA desaturase (SCD), an enzyme that controls synthesis of unsaturated fatty acids, as essential in breast and prostate cancer cells. SCD inhibition altered cellular lipid composition and impeded cell viability in the absence of exogenous lipids. SCD inhibition also altered cardiolipin composition, leading to the release of cytochrome C and induction of apoptosis. Furthermore, SCD was required for the generation of poly-unsaturated lipids in cancer cells grown in spheroid cultures, which resemble those found in tumour tissue. We also found that SCD mRNA and protein expression is elevated in human breast cancers and predicts poor survival in high-grade tumours. Finally, silencing of SCD in prostate orthografts efficiently blocked tumour growth and significantly increased animal survival.
Conclusions: Our data implicate lipid desaturation as an essential process for cancer cell survival and suggest that targeting SCD could efficiently limit tumour expansion, especially under the metabolically compromised conditions of the tumour microenvironment.
Keywords: Breast cancer; Lipid desaturation; Lipidomics; Prostate cancer; SCD.
Figures

References
Grants and funding
- MR/N020782/1/MRC_/Medical Research Council/United Kingdom
- 12011/CRUK_/Cancer Research UK/United Kingdom
- 11359/CRUK_/Cancer Research UK/United Kingdom
- P30 CA010815/CA/NCI NIH HHS/United States
- 16466/CRUK_/Cancer Research UK/United Kingdom
- 18974/CRUK_/Cancer Research UK/United Kingdom
- MR/J007986/1/MRC_/Medical Research Council/United Kingdom
- 18278/CRUK_/Cancer Research UK/United Kingdom
- EME/13/122/01/DH_/Department of Health/United Kingdom
- BBS/E/B/000C0417/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- NIHR/CS/009/009/DH_/Department of Health/United Kingdom
- G0700915/MRC_/Medical Research Council/United Kingdom
- 10337/CRUK_/Cancer Research UK/United Kingdom
- MC_EX_G0801762/MRC_/Medical Research Council/United Kingdom
- G0300648/MRC_/Medical Research Council/United Kingdom
- 15151/CRUK_/Cancer Research UK/United Kingdom
- BBS/E/B/000C0415/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources

