Insufficient disease inhibition by intrathecal rituximab in progressive multiple sclerosis
- PMID: 27042677
- PMCID: PMC4774261
- DOI: 10.1002/acn3.293
Insufficient disease inhibition by intrathecal rituximab in progressive multiple sclerosis
Abstract
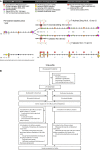
Objective: Inaccessibility of the inflammation compartmentalized to the central nervous system (CNS) may underlie the lack of efficacy of immunomodulatory treatments in progressive multiple sclerosis (MS). The double blind combination of Rituximab by IntraVenous and IntraThecAl injection versus placebo in patients with Low-Inflammatory SEcondary progressive MS (RIVITALISE; NCT01212094) trial was designed to answer: (1) Whether an induction dose of intravenous and intrathecal rituximab efficiently depletes CNS B cells? and (2) If so, whether this leads to global inhibition of CNS inflammation and slowing of CNS tissue destruction?
Methods: Patients aged 18-65 years were randomly assigned to rituximab or placebo. Protocol-stipulated interim analysis quantified the efficacy of B-cell depletion.
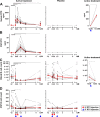
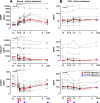
Results: The efficacy on cerebrospinal fluid (CSF) biomarkers failed to reach criteria for continuation of the trial. B-cell-related CSF biomarkers (sCD21 and B-cell activating factor) changed only in the active-treatment arm. While CSF B cells were killed robustly (median -79.71%, P = 0.0176), B cells in CNS tissue were depleted inadequately (~-10-20%, P < 0.0001). Consequently, the T-cell-specific CSF biomarker sCD27 decreased slightly (-10.97%, P = 0.0005), while axonal damage marker, neurofilament light chain did not change. Insufficient saturation of CD20, lack of lytic complement, and paucity of cytotoxic CD56(dim) NK cells contribute to decreased efficacy of rituximab in the CNS.
Interpretation: Biomarker studies reliably quantified complementary pharmacodynamic effects of rituximab in the CNS, exposed causes for poor efficacy and determined that RIVITALISE trial would be underpowered to measure efficacy on clinical outcomes. Identified mechanisms for poor efficacy are applicable to all CNS-inflammation targeting monoclonal antibodies.
Figures



References
-
- Stys PK, Zamponi GW, van Minnen J, Geurts JJ. Will the real multiple sclerosis please stand up? Nat Rev Neurosci 2012;13:507–514. - PubMed
-
- Magliozzi R, Howell O, Vora A, et al. Meningeal B‐cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain 2007;130(Pt 4):1089–1104. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

