Plasma neurofilament light chain predicts progression in progressive supranuclear palsy
- PMID: 27042681
- PMCID: PMC4774256
- DOI: 10.1002/acn3.290
Plasma neurofilament light chain predicts progression in progressive supranuclear palsy
Abstract
Objective: Blood-based biomarkers for neurodegenerative conditions could improve diagnosis and treatment development. Neurofilament light chain (NfL), a marker of axonal injury, is elevated in cerebrospinal fluid (CSF) of patients with progressive supranuclear palsy (PSP). The goal of this study was to determine the diagnostic and prognostic value of plasma NfL in patients with PSP.
Methods: Plasma NfL was measured with ultrasensitive digital immunoassay-based technology at baseline and 1-year follow-up in a pilot cohort of 15 PSP patients and 12 healthy controls, and a validation cohort of 147 PSP patients. Mixed linear models tested the ability of plasma NfL to predict neurological, cognitive and functional decline, and brain atrophy.
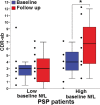
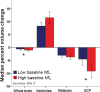
Results: Baseline mean plasma NfL levels were elevated in PSP patients (31 ± 4 pg/mL, vs. control, 17.5 ± 1 pg/mL, P < 0.05) and this difference persisted at follow-up. A cutoff value of 20 pg/mL related to the diagnosis of PSP with a sensitivity of 0.80 and specificity of 0.83 (positive likelihood ratio = 4.7 and a negative likelihood radio of 0.24). Patients with higher NfL levels had more severe neurological (PSPRS, -36.9% vs. -28.9%, P = 0.04), functional (SEADL, -38.2% vs. -20%, P = 0.03), and neuropsychological (RBANS, -23.9% vs. -12.3%, P = 001) deterioration over 1 year. Higher baseline NfL predicted greater whole-brain and superior cerebellar peduncle volume loss. Plasma and CSF NfL were significantly correlated (r = 0.74, P = 0.002).
Interpretation: Plasma NfL is elevated in PSP and could be of value as a biomarker both to assist clinical diagnosis and to monitor pharmacodynamic effects on the neurodegenerative process in clinical trials.
Figures



References
-
- Steele JC, Richardson JC, Olszewski J. Progressive supranuclear palsy. A heterogeneous degeneration involving the brain stem, basal ganglia and cerebellum with vertical gaze and pseudobulbar palsy, nuchal dystonia and dementia. Arch Neurol 1964;10:333–359. - PubMed
-
- Kawashima M, Miyake M, Kusumi M, et al. Prevalence of progressive supranuclear palsy in Yonago, Japan. Mov Disord 2004;19:1239–1240. - PubMed
-
- Nath U, Ben‐Shlomo Y, Thomson RG, et al. The prevalence of progressive supranuclear palsy (Steele‐Richardson‐Olszewski syndrome) in the UK. Brain 2001;124:1438–1449. - PubMed
-
- Golbe LI. Progressive supranuclear palsy. Semin Neurol 2014;34:151–159. - PubMed
-
- Hall S, Ohrfelt A, Constantinescu R, et al. Accuracy of a panel of 5 cerebrospinal fluid biomarkers in the differential diagnosis of patients with dementia and/or parkinsonian disorders. Arch Neurol 2012;69:1445–1452. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

