Scintillating Nanoparticles as Energy Mediators for Enhanced Photodynamic Therapy
- PMID: 27043181
- PMCID: PMC4846476
- DOI: 10.1021/acsnano.6b01401
Scintillating Nanoparticles as Energy Mediators for Enhanced Photodynamic Therapy
Abstract
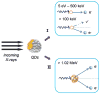
Achieving effective treatment of deep-seated tumors is a major challenge for traditional photodynamic therapy (PDT) due to difficulties in delivering light into the subsurface. Thanks to their great tissue penetration, X-rays hold the potential to become an ideal excitation source for activating photosensitizers (PS) that accumulate in deep tumor tissue. Recently, a wide variety of nanoparticles have been developed for this purpose. The nanoparticles are designed as carriers for loading various kinds of PSs and can facilitate the activation process by transferring energy harvested from X-ray irradiation to the loaded PS. In this review, we focus on recent developments of nanoscintillators with high energy transfer efficiency, their rational designs, as well as potential applications in next-generation PDT. Treatment of deep-seated tumors by using radioisotopes as an internal light source will also be discussed.
Keywords: Cerenkov radiation; X-ray activatable nanoparticles; cancer therapy; energy mediator; photodynamic therapy; photosensitizer; radiosensitizer; scintillating nanoparticles; scintillator.
Figures









Similar articles
-
Highly Efficient FRET System Capable of Deep Photodynamic Therapy Established on X-ray Excited Mesoporous LaF3:Tb Scintillating Nanoparticles.ACS Appl Mater Interfaces. 2015 Jun 10;7(22):12261-9. doi: 10.1021/acsami.5b03067. Epub 2015 May 26. ACS Appl Mater Interfaces. 2015. PMID: 25974980
-
Nanocomposite-Based Photodynamic Therapy Strategies for Deep Tumor Treatment.Small. 2015 Nov 25;11(44):5860-87. doi: 10.1002/smll.201501923. Epub 2015 Sep 23. Small. 2015. PMID: 26398119 Review.
-
Nanoscintillator-Mediated X-Ray Induced Photodynamic Therapy for Deep-Seated Tumors: From Concept to Biomedical Applications.Theranostics. 2020 Jan 1;10(3):1296-1318. doi: 10.7150/thno.41578. eCollection 2020. Theranostics. 2020. PMID: 31938066 Free PMC article. Review.
-
Two-photon excitation nanoparticles for photodynamic therapy.Chem Soc Rev. 2016 Dec 21;45(24):6725-6741. doi: 10.1039/c6cs00442c. Epub 2016 Oct 5. Chem Soc Rev. 2016. PMID: 27711672 Review.
-
Development of a functionalized UV-emitting nanocomposite for the treatment of cancer using indirect photodynamic therapy.J Nanobiotechnology. 2018 Feb 27;16(1):19. doi: 10.1186/s12951-018-0344-3. J Nanobiotechnology. 2018. PMID: 29482561 Free PMC article.
Cited by
-
Treating Deep-Seated Tumors with Radiodynamic Therapy: Progress and Perspectives.Pharmaceutics. 2024 Aug 28;16(9):1135. doi: 10.3390/pharmaceutics16091135. Pharmaceutics. 2024. PMID: 39339173 Free PMC article. Review.
-
Optical and x-ray technology synergies enabling diagnostic and therapeutic applications in medicine.J Biomed Opt. 2018 Oct;23(12):1-17. doi: 10.1117/1.JBO.23.12.121610. J Biomed Opt. 2018. PMID: 30350489 Free PMC article. Review.
-
Spatially Specific Liposomal Cancer Therapy Triggered by Clinical External Sources of Energy.Pharmaceutics. 2019 Mar 16;11(3):125. doi: 10.3390/pharmaceutics11030125. Pharmaceutics. 2019. PMID: 30884786 Free PMC article. Review.
-
Application of Mitochondrially Targeted Nanoconstructs to Neoadjuvant X-ray-Induced Photodynamic Therapy for Rectal Cancer.ACS Cent Sci. 2020 May 27;6(5):715-726. doi: 10.1021/acscentsci.9b01121. Epub 2020 Apr 24. ACS Cent Sci. 2020. PMID: 32490188 Free PMC article.
-
Nanocomposites for X-Ray Photodynamic Therapy.Int J Mol Sci. 2020 Jun 3;21(11):4004. doi: 10.3390/ijms21114004. Int J Mol Sci. 2020. PMID: 32503329 Free PMC article. Review.
References
-
- DeRosa MC, Crutchley RJ. Photosensitized Singlet Oxygen and Its Applications. Coord Chem Rev. 2002;233:351–371.
-
- Jacques SL. Optical Properties of Biological Tissues: A Review. Phys Med Biol. 2013;58:R37–61. - PubMed
-
- Ethirajan M, Chen Y, Joshi P, Pandey RK. The Role of Porphyrin Chemistry in Tumor Imaging and Photodynamic Therapy. Chem Soc Rev. 2011;40:340–362. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

