A GABAergic nigrotectal pathway for coordination of drinking behavior
- PMID: 27043290
- PMCID: PMC5014542
- DOI: 10.1038/nn.4285
A GABAergic nigrotectal pathway for coordination of drinking behavior
Abstract
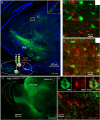
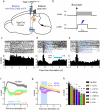
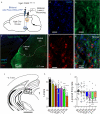
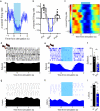
The contribution of basal ganglia outputs to consummatory behavior remains poorly understood. We recorded from the substantia nigra pars reticulata (SNR), the major basal ganglia output nucleus, during self-initiated drinking in mice. The firing rates of many lateral SNR neurons were time-locked to individual licks. These neurons send GABAergic projections to the deep layers of the orofacial region of the lateral tectum (superior colliculus, SC). Many tectal neurons were also time-locked to licking, but their activity was usually in antiphase with that of SNR neurons, suggesting inhibitory nigrotectal projections. We used optogenetics to selectively activate the GABAergic nigrotectal afferents in the deep layers of the SC. Photo-stimulation of the nigrotectal projections transiently inhibited the activity of the lick-related tectal neurons, disrupted their licking-related oscillatory pattern and suppressed self-initiated drinking. These results demonstrate that GABAergic nigrotectal projections have a crucial role in coordinating drinking behavior.
Figures



References
-
- Fowler SC, Mortell C. Low doses of haloperidol interfere with rat tongue extensions during licking: a quantitative analysis. Behav Neurosci. 1992;106:386–395. - PubMed
-
- Nakamura S, Muramatsu S, Yoshida M. Role of the basal ganglia in manifestation of rhythmical jaw movement in rats. Brain research. 1990;535:335–338. - PubMed
-
- Travers JB, Dinardo LA, Karimnamazi H. Motor and premotor mechanisms of licking. Neurosci Biobehav Rev. 1997;21:631–647. - PubMed
-
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–271. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

