Silymarin Prevents Restraint Stress-Induced Acute Liver Injury by Ameliorating Oxidative Stress and Reducing Inflammatory Response
- PMID: 27043523
- PMCID: PMC6274117
- DOI: 10.3390/molecules21040443
Silymarin Prevents Restraint Stress-Induced Acute Liver Injury by Ameliorating Oxidative Stress and Reducing Inflammatory Response
Abstract
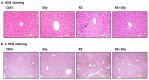
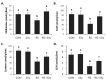
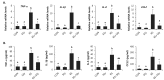
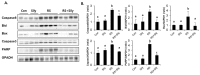
Silymarin is a flavonoid extracted from the milk thistle Silybum marianum. It has been reported to prevent liver injuries induced by various chemicals or toxins. Our recent study suggested that silymarin induces hepatic synthesis of glutathione by increasing cysteine availability, which may consequently contribute to increased antioxidant capacity of the liver. In the present study, we investigated the effects of silymarin on acute liver injury induced by restraint stress. Silymarin (100 mg/kg) was orally administered to BALB/c mice every 12 h (3 times in total). After the last dose, mice were subjected to restraint stress for 6 h, and serum levels of aspartate and alanine aminotransferases, and hepatic levels of lipid peroxidation were determined. Hepatic levels of sulfur-containing metabolites such as methionine, S-adenosylmethionine, cysteine, and glutathione were also measured. The level of pro-inflammatory mediators in both liver and serum was determined. To study the mechanism of the effects of silymarin, we assessed Jun N-terminal kinase (JNK) activation and apoptotic signaling. Restraint stress induced severe oxidative stress and increased mRNA levels of pro-inflammatory mediators; both effects of restraint stress were significantly inhibited by silymarin. Moreover, administration of silymarin significantly prevented acute liver injury induced by restraint stress by blocking JNK activation and subsequently apoptotic signaling. In conclusion, these results suggest that the inhibition of restraint stress-induced liver injury by silymarin is due at least in part to its anti-oxidant activity and its ability to suppress the inflammatory response.
Keywords: acute liver injury; inflammation; oxidative stress; restraint stress; silymarin.
Conflict of interest statement
The authors declare that they have no financial or nonfinancial competing interests.
Figures
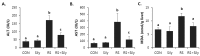

References
-
- Cao H.J., Tan R.R., He R.R., Tang L.P., Wang X.L., Yao N., Duan W.J., Hu Y.A., Yao X.S., Kurihara H. Sarcandra glabra extract reduces the susceptibility and severity of influenza in restraint-stressed mice. Evid. Based Complement. Altern. Med. 2012;2012 doi: 10.1155/2012/236539. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

