Outcomes in Adults With Acute Liver Failure Between 1998 and 2013: An Observational Cohort Study
- PMID: 27043883
- PMCID: PMC5526039
- DOI: 10.7326/M15-2211
Outcomes in Adults With Acute Liver Failure Between 1998 and 2013: An Observational Cohort Study
Abstract
Background: Acute liver failure (ALF) is a rare syndrome of severe, rapid-onset hepatic dysfunction-without prior advanced liver disease-that is associated with high morbidity and mortality. Intensive care and liver transplantation provide support and rescue, respectively.
Objective: To determine whether changes in causes, disease severity, treatment, or 21-day outcomes have occurred in recent years among adult patients with ALF referred to U.S. tertiary care centers.
Design: Prospective observational cohort study. (ClinicalTrials .gov: NCT00518440).
Setting: 31 liver disease and transplant centers in the United States.
Patients: Consecutively enrolled patients-without prior advanced liver disease-with ALF (n = 2070).
Measurements: Clinical features, treatment, and 21-day outcomes were compared over time annually for trends and were also stratified into two 8-year periods (1998 to 2005 and 2006 to 2013).
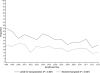
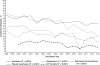
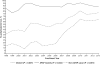
Results: Overall clinical characteristics, disease severity, and distribution of causes remained similar throughout the study period. The 21-day survival rates increased between the two 8-year periods (overall, 67.1% vs. 75.3%; transplant-free survival [TFS], 45.1% vs. 56.2%; posttransplantation survival, 88.3% vs. 96.3% [P < 0.010 for each]). Reductions in red blood cell infusions (44.3% vs. 27.6%), plasma infusions (65.2% vs. 47.1%), mechanical ventilation (65.7% vs. 56.1%), and vasopressors (34.9% vs. 27.8%) were observed, as well as increased use of N-acetylcysteine (48.9% vs. 69.3% overall; 15.8% vs. 49.4% [P < 0.001] in patients with ALF not due to acetaminophen toxicity). When examined longitudinally, overall survival and TFS increased throughout the 16-year period.
Limitations: The duration of enrollment, the number of patients enrolled, and possibly the approaches to care varied among participating sites. The results may not be generalizable beyond such specialized centers.
Conclusion: Although characteristics and severity of ALF changed little over 16 years, overall survival and TFS improved significantly. The effects of specific changes in intensive care practice on survival warrant further study.
Primary funding source: National Institutes of Health.
Figures


Comment in
-
Acute liver failure in adults.Turk J Gastroenterol. 2019 Oct;30(10):938-939. doi: 10.5152/tjg.2019.260919. Turk J Gastroenterol. 2019. PMID: 31625941 Free PMC article. No abstract available.
References
-
- Ichai P, Samuel D. Etiology and prognosis of fulminant hepatitis in adults. Liver Transpl. 2008;14(Suppl 2):S67–79. - PubMed
-
- Ostapowicz G, Fontana RJ, Schiødt FV, Larson A, Davern TJ, Han SH, et al. U.S. Acute Liver Failure Study Group. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947–54. - PubMed
-
- Bernal W, Hyyrylainen A, Gera A, Audimoolam VK, McPhail MJ, Auzinger G, et al. Lessons from look-back in acute liver failure? A single centre experience of 3300 patients. J Hepatol. 2013;59:74–80. - PubMed
-
- Stravitz RT, Kramer AH, Davern T, Shaikh AO, Caldwell SH, Mehta RL, et al. Acute Liver Failure Study Group. Intensive care of patients with acute liver failure: recommendations of the U.S. Acute Liver Failure Study Group. Crit Care Med. 2007;35:2498–508. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
