Real-time monitoring of metabolic function in liver-on-chip microdevices tracks the dynamics of mitochondrial dysfunction
- PMID: 27044092
- PMCID: PMC4843487
- DOI: 10.1073/pnas.1522556113
Real-time monitoring of metabolic function in liver-on-chip microdevices tracks the dynamics of mitochondrial dysfunction
Abstract
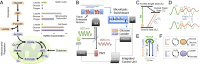
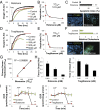
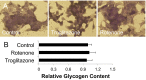
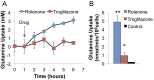
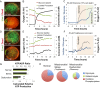
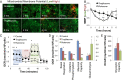
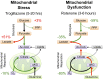
Microfluidic organ-on-a-chip technology aims to replace animal toxicity testing, but thus far has demonstrated few advantages over traditional methods. Mitochondrial dysfunction plays a critical role in the development of chemical and pharmaceutical toxicity, as well as pluripotency and disease processes. However, current methods to evaluate mitochondrial activity still rely on end-point assays, resulting in limited kinetic and prognostic information. Here, we present a liver-on-chip device capable of maintaining human tissue for over a month in vitro under physiological conditions. Mitochondrial respiration was monitored in real time using two-frequency phase modulation of tissue-embedded phosphorescent microprobes. A computer-controlled microfluidic switchboard allowed contiguous electrochemical measurements of glucose and lactate, providing real-time analysis of minute shifts from oxidative phosphorylation to anaerobic glycolysis, an early indication of mitochondrial stress. We quantify the dynamics of cellular adaptation to mitochondrial damage and the resulting redistribution of ATP production during rotenone-induced mitochondrial dysfunction and troglitazone (Rezulin)-induced mitochondrial stress. We show troglitazone shifts metabolic fluxes at concentrations previously regarded as safe, suggesting a mechanism for its observed idiosyncratic effect. Our microfluidic platform reveals the dynamics and strategies of cellular adaptation to mitochondrial damage, a unique advantage of organ-on-chip technology.
Keywords: liver tissue engineering; microfluidics; organ-on-a-chip; toxicology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Allen JW, Bhatia SN. Formation of steady-state oxygen gradients in vitro: Application to liver zonation. Biotechnol Bioeng. 2003;82(3):253–262. - PubMed
-
- Khademhosseini A, et al. Cell docking inside microwells within reversibly sealed microfluidic channels for fabricating multiphenotype cell arrays. Lab Chip. 2005;5(12):1380–1386. - PubMed
-
- Sivaraman A, et al. A microscale in vitro physiological model of the liver: Predictive screens for drug metabolism and enzyme induction. Curr Drug Metab. 2005;6(6):569–591. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

