Accumulation of formamide in hydrothermal pores to form prebiotic nucleobases
- PMID: 27044100
- PMCID: PMC4843465
- DOI: 10.1073/pnas.1600275113
Accumulation of formamide in hydrothermal pores to form prebiotic nucleobases
Abstract
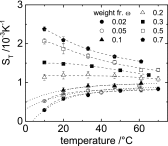
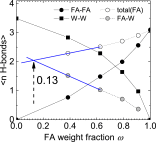
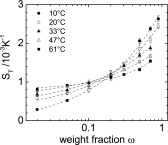
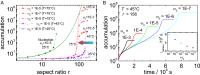
Formamide is one of the important compounds from which prebiotic molecules can be synthesized, provided that its concentration is sufficiently high. For nucleotides and short DNA strands, it has been shown that a high degree of accumulation in hydrothermal pores occurs, so that temperature gradients might play a role in the origin of life [Baaske P, et al. (2007)Proc Natl Acad Sci USA104(22):9346-9351]. We show that the same combination of thermophoresis and convection in hydrothermal pores leads to accumulation of formamide up to concentrations where nucleobases are formed. The thermophoretic properties of aqueous formamide solutions are studied by means of Infrared Thermal Diffusion Forced Rayleigh Scattering. These data are used in numerical finite element calculations in hydrothermal pores for various initial concentrations, ambient temperatures, and pore sizes. The high degree of formamide accumulation is due to an unusual temperature and concentration dependence of the thermophoretic behavior of formamide. The accumulation fold in part of the pores increases strongly with increasing aspect ratio of the pores, and saturates to highly concentrated aqueous formamide solutions of ∼85 wt% at large aspect ratios. Time-dependent studies show that these high concentrations are reached after 45-90 d, starting with an initial formamide weight fraction of[Formula: see text]wt % that is typical for concentrations in shallow lakes on early Earth.
Keywords: concentration problem; hydrothermal vents; molecular evolution; origin-of-life problem; thermophoresis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Extreme accumulation of nucleotides in simulated hydrothermal pore systems.Proc Natl Acad Sci U S A. 2007 May 29;104(22):9346-51. doi: 10.1073/pnas.0609592104. Epub 2007 May 9. Proc Natl Acad Sci U S A. 2007. PMID: 17494767 Free PMC article.
-
Stability of 2',3' and 3',5' cyclic nucleotides in formamide and in water: a theoretical insight into the factors controlling the accumulation of nucleic acid building blocks in a prebiotic pool.Phys Chem Chem Phys. 2017 Jan 18;19(3):1817-1825. doi: 10.1039/c6cp07993h. Phys Chem Chem Phys. 2017. PMID: 28000820
-
Formamide-based synthesis of nucleobases by metal(II) octacyanomolybdate(IV): implication in prebiotic chemistry.Astrobiology. 2014 Sep;14(9):769-79. doi: 10.1089/ast.2014.1187. Epub 2014 Sep 5. Astrobiology. 2014. PMID: 25192494
-
Emergence of the First Catalytic Oligonucleotides in a Formamide-Based Origin Scenario.Chemistry. 2016 Mar 7;22(11):3572-86. doi: 10.1002/chem.201503906. Epub 2016 Jan 25. Chemistry. 2016. PMID: 26807661 Review.
-
Formamide chemistry and the origin of informational polymers.Chem Biodivers. 2007 Apr;4(4):694-720. doi: 10.1002/cbdv.200790059. Chem Biodivers. 2007. PMID: 17443884 Review.
Cited by
-
Thermophoretic migration of vesicles depends on mean temperature and head group chemistry.Nat Commun. 2017 May 17;8:15351. doi: 10.1038/ncomms15351. Nat Commun. 2017. PMID: 28513597 Free PMC article.
-
Considering planetary environments in origin of life studies.Nat Commun. 2018 Dec 12;9(1):5170. doi: 10.1038/s41467-018-07493-3. Nat Commun. 2018. PMID: 30538232 Free PMC article.
-
The Formation of RNA Pre-Polymers in the Presence of Different Prebiotic Mineral Surfaces Studied by Molecular Dynamics Simulations.Life (Basel). 2022 Dec 30;13(1):112. doi: 10.3390/life13010112. Life (Basel). 2022. PMID: 36676060 Free PMC article.
-
Chemomimesis and Molecular Darwinism in Action: From Abiotic Generation of Nucleobases to Nucleosides and RNA.Life (Basel). 2018 Jun 20;8(2):24. doi: 10.3390/life8020024. Life (Basel). 2018. PMID: 29925796 Free PMC article. Review.
-
Biomimetic mineral self-organization from silica-rich spring waters.Sci Adv. 2017 Mar 17;3(3):e1602285. doi: 10.1126/sciadv.1602285. eCollection 2017 Mar. Sci Adv. 2017. PMID: 28345049 Free PMC article.
References
-
- Harada K. Formation of amino-acids by thermal decomposition of formamide−oligomerization of hydrogen cyanide. Nature. 1967;214(5087):479–480.
-
- Miyakawa S, Cleaves HJ, Miller SL. The cold origin of life: A. Implications based on the hydrolytic stabilities of hydrogen cyanide and formamide. Orig Life Evol Biosph. 2002;32(3):195–208. - PubMed
-
- Saladino R, Crestini C, Pino S, Costanzo G, Di Mauro E. Formamide and the origin of life. Phys Life Rev. 2012;9(1):84–104. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

