Proteus mirabilis fimbriae- and urease-dependent clusters assemble in an extracellular niche to initiate bladder stone formation
- PMID: 27044107
- PMCID: PMC4843424
- DOI: 10.1073/pnas.1601720113
Proteus mirabilis fimbriae- and urease-dependent clusters assemble in an extracellular niche to initiate bladder stone formation
Abstract
The catheter-associated uropathogenProteus mirabilisfrequently causes urinary stones, but little has been known about the initial stages of bladder colonization and stone formation. We found thatP. mirabilisrapidly invades the bladder urothelium, but generally fails to establish an intracellular niche. Instead, it forms extracellular clusters in the bladder lumen, which form foci of mineral deposition consistent with development of urinary stones. These clusters elicit a robust neutrophil response, and we present evidence of neutrophil extracellular trap generation during experimental urinary tract infection. We identified two virulence factors required for cluster development: urease, which is required for urolithiasis, and mannose-resistantProteus-like fimbriae. The extracellular cluster formation byP. mirabilisstands in direct contrast to uropathogenicEscherichia coli, which readily formed intracellular bacterial communities but not luminal clusters or urinary stones. We propose that extracellular clusters are a key mechanism ofP. mirabilissurvival and virulence in the bladder.
Keywords: Proteus mirabilis; bladder stones; fimbriae; urease; urinary tract infection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



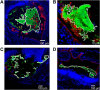


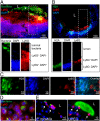


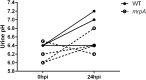

Comment in
-
Re: Proteus mirabilis Fimbriae- and Urease-Dependent Clusters Assemble in an Extracellular Niche to Initiate Bladder Stone Formation.J Urol. 2017 May;197(5):1298. doi: 10.1016/j.juro.2017.02.012. Epub 2017 Feb 9. J Urol. 2017. PMID: 29539914 No abstract available.
Similar articles
-
From Catheter to Kidney Stone: The Uropathogenic Lifestyle of Proteus mirabilis.Trends Microbiol. 2017 Apr;25(4):304-315. doi: 10.1016/j.tim.2016.11.015. Epub 2016 Dec 22. Trends Microbiol. 2017. PMID: 28017513 Free PMC article. Review.
-
Visualization of Proteus mirabilis within the matrix of urease-induced bladder stones during experimental urinary tract infection.Infect Immun. 2002 Jan;70(1):389-94. doi: 10.1128/IAI.70.1.389-394.2002. Infect Immun. 2002. PMID: 11748205 Free PMC article.
-
In vivo phase variation of MR/P fimbrial gene expression in Proteus mirabilis infecting the urinary tract.Mol Microbiol. 1997 Mar;23(5):1009-19. doi: 10.1046/j.1365-2958.1997.2791645.x. Mol Microbiol. 1997. PMID: 9076737
-
Use of green fluorescent protein to assess urease gene expression by uropathogenic Proteus mirabilis during experimental ascending urinary tract infection.Infect Immun. 1998 Jan;66(1):330-5. doi: 10.1128/IAI.66.1.330-335.1998. Infect Immun. 1998. PMID: 9423875 Free PMC article.
-
Virulence determinants of uropathogenic Escherichia coli and Proteus mirabilis.Kidney Int Suppl. 1994 Nov;47:S129-36. Kidney Int Suppl. 1994. PMID: 7869662 Review.
Cited by
-
Proteus mirabilis Urease: Unsuspected Non-Enzymatic Properties Relevant to Pathogenicity.Int J Mol Sci. 2021 Jul 4;22(13):7205. doi: 10.3390/ijms22137205. Int J Mol Sci. 2021. PMID: 34281258 Free PMC article.
-
Tamm-Horsfall protein augments neutrophil NETosis during urinary tract infection.JCI Insight. 2025 Jan 9;10(1):e180024. doi: 10.1172/jci.insight.180024. JCI Insight. 2025. PMID: 39589812 Free PMC article.
-
Muting Bacterial Communication: Evaluation of Prazosin Anti-Quorum Sensing Activities against Gram-Negative Bacteria Pseudomonas aeruginosa, Proteus mirabilis, and Serratia marcescens.Biology (Basel). 2022 Sep 13;11(9):1349. doi: 10.3390/biology11091349. Biology (Basel). 2022. PMID: 36138828 Free PMC article.
-
Secretory System Components as Potential Prophylactic Targets for Bacterial Pathogens.Biomolecules. 2021 Jun 15;11(6):892. doi: 10.3390/biom11060892. Biomolecules. 2021. PMID: 34203937 Free PMC article. Review.
-
Proteus mirabilis UreR coordinates cellular functions required for urease activity.J Bacteriol. 2024 Apr 18;206(4):e0003124. doi: 10.1128/jb.00031-24. Epub 2024 Mar 27. J Bacteriol. 2024. PMID: 38534115 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

