In vitro and in vivo activity of melflufen (J1)in lymphoma
- PMID: 27044263
- PMCID: PMC4820867
- DOI: 10.1186/s12885-016-2299-9
In vitro and in vivo activity of melflufen (J1)in lymphoma
Abstract
Background: Melphalan has been used in the treatment of various hematologic malignancies for almost 60 years. Today it is part of standard therapy for multiple myeloma and also as part of myeloablative regimens in association with autologous allogenic stem cell transplantation. Melflufen (melphalan flufenamide ethyl ester, previously called J1) is an optimized derivative of melphalan providing targeted delivery of active metabolites to cells expressing aminopeptidases. The activity of melflufen has compared favorably with that of melphalan in a series of in vitro and in vivo experiments performed preferentially on different solid tumor models and multiple myeloma. Melflufen is currently being evaluated in a clinical phase I/II trial in relapsed or relapsed and refractory multiple myeloma.
Methods: Cytotoxicity of melflufen was assayed in lymphoma cell lines and in primary tumor cells with the Fluorometric Microculture Cytotoxicity Assay and cell cycle analyses was performed in two of the cell lines. Melflufen was also investigated in a xenograft model with subcutaneous lymphoma cells inoculated in mice.
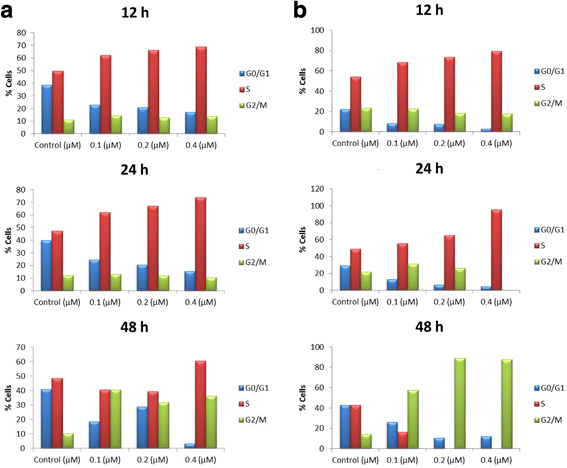
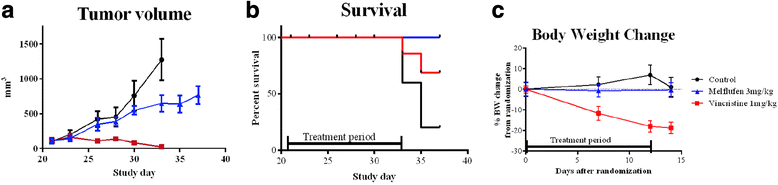
Results: Melflufen showed activity with cytotoxic IC50-values in the submicromolar range (0.011-0.92 μM) in the cell lines, corresponding to a mean of 49-fold superiority (p < 0.001) in potency vs. melphalan. In the primary cultures melflufen yielded slightly lower IC50-values (2.7 nM to 0.55 μM) and an increased ratio vs. melphalan (range 13-455, average 108, p < 0.001). Treated cell lines exhibited a clear accumulation in the G2/M-phase of the cell cycle. Melflufen also showed significant activity and no, or minimal side effects in the xenografted animals.
Conclusion: This study confirms previous reports of a targeting related potency superiority of melflufen compared to that of melphalan. Melflufen was active in cell lines and primary cultures of lymphoma cells, as well as in a xenograft model in mice and appears to be a candidate for further evaluation in the treatment of this group of malignant diseases.
Keywords: Alkylating agents; Cancer therapeutics; J1; Melflufen; Prodrug.
Figures


References
-
- Bergel F, Stock JA. Cyto-active Amino-acid and Peptide Derivatives. Part I. Substituted Phenylalanines. J Chem Soc. 1954: 2409–17
-
- Bergel F, Stock JA. Cytotoxic alpha amino acids and peptides. Br Emp Cancer Camp Annu Rep. 1951;31:6–7.
-
- Bergel F, Burnop VCE Stock JA. Cyto-active Amino-acids and Peptides. Part II. Resolution of Substituted Phenylalanines and Synthesis of p-Di-(2-chloroethyl)amino-DL-phenyl[ß-14C]alanine. J Chem Soc. 1955:1223-9
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

