Trim37-deficient mice recapitulate several features of the multi-organ disorder Mulibrey nanism
- PMID: 27044324
- PMCID: PMC4874348
- DOI: 10.1242/bio.016246
Trim37-deficient mice recapitulate several features of the multi-organ disorder Mulibrey nanism
Abstract
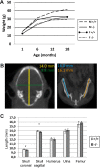
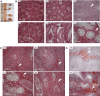
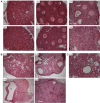
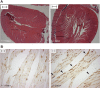
Mulibrey nanism (MUL) is a rare autosomal recessive multi-organ disorder characterized by severe prenatal-onset growth failure, infertility, cardiopathy, risk for tumors, fatty liver, and type 2 diabetes. MUL is caused by loss-of-function mutations in TRIM37, which encodes an E3 ubiquitin ligase belonging to the tripartite motif (TRIM) protein family and having both peroxisomal and nuclear localization. We describe a congenic Trim37 knock-out mouse (Trim37(-/-)) model for MUL. Trim37(-/-) mice were viable and had normal weight development until approximately 12 months of age, after which they started to manifest increasing problems in wellbeing and weight loss. Assessment of skeletal parameters with computer tomography revealed significantly smaller skull size, but no difference in the lengths of long bones in Trim37(-/-) mice as compared with wild-type. Both male and female Trim37(-/-) mice were infertile, the gonads showing germ cell aplasia, hilus and Leydig cell hyperplasia and accumulation of lipids in and around Leydig cells. Male Trim37(-/-) mice had elevated levels of follicle-stimulating and luteinizing hormones, but maintained normal levels of testosterone. Six-month-old Trim37(-/-) mice had elevated fasting blood glucose and low fasting serum insulin levels. At 1.5 years Trim37(-/-) mice showed non-compaction cardiomyopathy, hepatomegaly, fatty liver and various tumors. The amount and morphology of liver peroxisomes seemed normal in Trim37(-/-) mice. The most consistently seen phenotypes in Trim37(-/-) mice were infertility and the associated hormonal findings, whereas there was more variability in the other phenotypes observed. Trim37(-/-) mice recapitulate several features of the human MUL disease and thus provide a good model to study disease pathogenesis related to TRIM37 deficiency, including infertility, non-alcoholic fatty liver disease, cardiomyopathy and tumorigenesis.
Keywords: Cardiomyopathy; Fatty liver; Growth failure; Infertility; Mulibrey nanism; Tumorigenesis.
© 2016. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures


Similar articles
-
TRIMming down to TRIM37: Relevance to Inflammation, Cardiovascular Disorders, and Cancer in MULIBREY Nanism.Int J Mol Sci. 2018 Dec 24;20(1):67. doi: 10.3390/ijms20010067. Int J Mol Sci. 2018. PMID: 30586926 Free PMC article. Review.
-
TRIM37 defective in mulibrey nanism is a novel RING finger ubiquitin E3 ligase.Exp Cell Res. 2005 Aug 1;308(1):146-55. doi: 10.1016/j.yexcr.2005.04.001. Exp Cell Res. 2005. PMID: 15885686
-
Novel mutations in the TRIM37 gene in Mulibrey Nanism.Hum Mutat. 2004 May;23(5):522. doi: 10.1002/humu.9233. Hum Mutat. 2004. PMID: 15108285
-
Tissue expression of the mulibrey nanism-associated Trim37 protein in embryonic and adult mouse tissues.Histochem Cell Biol. 2006 Sep;126(3):325-34. doi: 10.1007/s00418-006-0162-9. Epub 2006 Mar 3. Histochem Cell Biol. 2006. PMID: 16514549
-
TRIM37: a critical orchestrator of centrosome function.Cell Cycle. 2021 Dec;20(23):2443-2451. doi: 10.1080/15384101.2021.1988289. Epub 2021 Oct 21. Cell Cycle. 2021. PMID: 34672905 Free PMC article. Review.
Cited by
-
TRIMming down to TRIM37: Relevance to Inflammation, Cardiovascular Disorders, and Cancer in MULIBREY Nanism.Int J Mol Sci. 2018 Dec 24;20(1):67. doi: 10.3390/ijms20010067. Int J Mol Sci. 2018. PMID: 30586926 Free PMC article. Review.
-
TRIM37, a novel E3 ligase for PEX5-mediated peroxisomal matrix protein import.J Cell Biol. 2017 Sep 4;216(9):2843-2858. doi: 10.1083/jcb.201611170. Epub 2017 Jul 19. J Cell Biol. 2017. PMID: 28724525 Free PMC article.
-
CD4+ T Cell Defects in a Mulibrey Patient With Specific TRIM37 Mutations.Front Immunol. 2020 Sep 18;11:1742. doi: 10.3389/fimmu.2020.01742. eCollection 2020. Front Immunol. 2020. PMID: 33042106 Free PMC article.
-
The TRIM37 variants in Mulibrey nanism patients paralyze follicular helper T cell differentiation.Cell Discov. 2023 Aug 1;9(1):82. doi: 10.1038/s41421-023-00561-z. Cell Discov. 2023. PMID: 37528081 Free PMC article.
-
Histone H2A Monoubiquitination in Neurodevelopmental Disorders.Trends Genet. 2017 Aug;33(8):566-578. doi: 10.1016/j.tig.2017.06.002. Epub 2017 Jun 29. Trends Genet. 2017. PMID: 28669576 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

