Short peptide based nanotubes capable of effective curcumin delivery for treating drug resistant malaria
- PMID: 27044333
- PMCID: PMC4820878
- DOI: 10.1186/s12951-016-0179-8
Short peptide based nanotubes capable of effective curcumin delivery for treating drug resistant malaria
Abstract
Background: Curcumin (Ccm) has shown immense potential as an antimalarial agent; however its low solubility and less bioavailability attenuate the in vivo efficacy of this potent compound. In order to increase Ccm's bioavailability, a number of organic/inorganic polymer based nanoparticles have been investigated. However, most of the present day nano based delivery systems pose a conundrum with respect to their complex synthesis procedures, poor in vivo stability and toxicity issues. Peptides due to their high biocompatibility could act as excellent materials for the synthesis of nanoparticulate drug delivery systems. Here, we have investigated dehydrophenylalanine (ΔPhe) di-peptide based self-assembled nanoparticles for the efficient delivery of Ccm as an antimalarial agent. The self-assembly and curcumin loading capacity of different ΔPhe dipeptides, phenylalanine-α,β-dehydrophenylalanine (FΔF), arginine-α,β-dehydrophenylalanine (RΔF), valine-α,β-dehydrophenylalanine (VΔF) and methonine-α,β-dehydrophenylalanine (MΔF) were investigated for achieving enhanced and effective delivery of the compound for potential anti-malarial therapy.
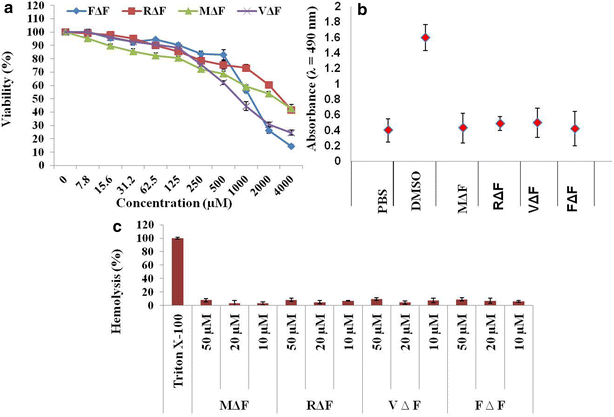
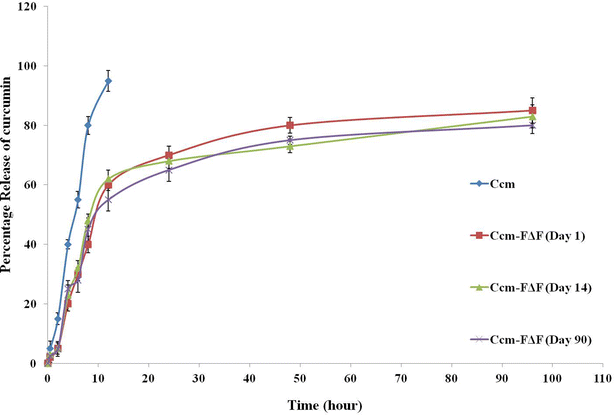
Results: FΔF, RΔF, VΔF and MΔF peptides formed different types of nanoparticles like nanotubes and nanovesicles under similar assembling conditions. Out of these, F∆F nanotubes showed maximum curcumin loading capacity of almost 68 % W/W. Ccm loaded F∆F nanotubes (Ccm-F∆F) showed comparatively higher (IC50, 3.0 µM) inhibition of Plasmodium falciparum (Indo strain) as compared to free Ccm (IC50, 13 µM). Ccm-F∆F nano formulation further demonstrated higher inhibition of parasite growth in malaria infected mice as compared to free Ccm. The dipeptide nanoparticles were highly biocompatible and didn't show any toxic effect on mammalian cell lines and normal blood cells.
Conclusion: This work provides a proof of principle of using highly biocompatible short peptide based nanoparticles for entrapment and in vivo delivery of Ccm leading to an enhancement in its efficacy as an antimalarial agent.
Keywords: Antimalarial; Curcumin; Nanotubes; Peptide; Self-assembly.
Figures







References
-
- Report WM. Malaria media center. In: Organization WH, editor. Geneva: WHO; 2014.
-
- Lehane AM, Kirk K. Efflux of a range of antimalarial drugs and ‘chloroquine resistance reversers’ from the digestive vacuole in malaria parasites with mutant PfCRT. Mol Microbiol. 2010;77(4):1039–1051. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

