VCAM-1+ placenta chorionic villi-derived mesenchymal stem cells display potent pro-angiogenic activity
- PMID: 27044487
- PMCID: PMC4820943
- DOI: 10.1186/s13287-016-0297-0
VCAM-1+ placenta chorionic villi-derived mesenchymal stem cells display potent pro-angiogenic activity
Abstract
Introduction: Mesenchymal stem cells (MSCs) represent a heterogeneous cell population that is promising for regenerative medicine. The present study was designed to assess whether VCAM-1 can be used as a marker of MSC subpopulation with superior angiogenic potential.
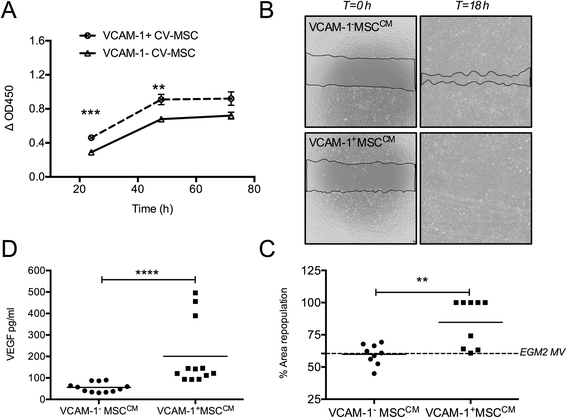
Methods: MSCs were isolated from placenta chorionic villi (CV). The VCAM-1(+/-) CV-MSCs population were separated by Flow Cytometry and subjected to a comparative analysis for their angiogenic properties including angiogenic genes expression, vasculo-angiogenic abilities on Matrigel in vitro and in vivo, angiogenic paracrine activities, cytokine array, and therapeutic angiogenesis in vascular ischemic diseases.
Results: Angiogenic genes, including HGF, ANG, IL8, IL6, VEGF-A, TGFβ, MMP2 and bFGF, were up-regulated in VCAM-1(+)CV-MSCs. Consistently, angiogenic cytokines especially HGF, IL8, angiogenin, angiopoitin-2, μPAR, CXCL1, IL-1β, IL-1α, CSF2, CSF3, MCP-3, CTACK, and OPG were found to be significantly increased in VCAM-1(+) CV-MSCs. Moreover, VCAM-1(+)CV-MSCs showed remarkable vasculo-angiogenic abilities by angiogenesis analysis with Matrigel in vitro and in vivo and the conditioned medium of VCAM-1(+) CV-MSCs exerted markedly pro-proliferative and pro-migratory effects on endothelial cells compared to VCAM-1(-)CV-MSCs. Finally, transplantation of VCAM-1(+)CV-MSCs into the ischemic hind limb of BALB/c nude mice resulted in a significantly functional improvement in comparison with VCAM-1(-)CV-MSCs transplantation.
Conclusions: VCAM-1(+)CV-MSCs possessed a favorable angiogenic paracrine activity and displayed therapeutic efficacy on hindlimb ischemia. Our results suggested that VCAM-1(+)CV-MSCs may represent an important subpopulation of MSC for efficient therapeutic angiogenesis.
Keywords: Angiogenesis; Mesenchymal stem cells; Paracrine; Placenta; Vascular cell adhesion molecule-1 (CD106).
Figures





Similar articles
-
Heterogeneity of proangiogenic features in mesenchymal stem cells derived from bone marrow, adipose tissue, umbilical cord, and placenta.Stem Cell Res Ther. 2016 Nov 10;7(1):163. doi: 10.1186/s13287-016-0418-9. Stem Cell Res Ther. 2016. PMID: 27832825 Free PMC article.
-
New insights into the heterogeneity and functional diversity of human mesenchymal stem cells.Biomed Mater Eng. 2017;28(s1):S29-S45. doi: 10.3233/BME-171622. Biomed Mater Eng. 2017. PMID: 28372276
-
Self-assembled GFFYK peptide hydrogel enhances the therapeutic efficacy of mesenchymal stem cells in a mouse hindlimb ischemia model.Acta Biomater. 2019 Feb;85:94-105. doi: 10.1016/j.actbio.2018.12.015. Epub 2018 Dec 11. Acta Biomater. 2019. PMID: 30550934
-
Mesenchymal Stromal Cells (MSCs): A Promising Tool for Cell-Based Angiogenic Therapy.Curr Gene Ther. 2021;21(5):382-405. doi: 10.2174/1566523221666210917114353. Curr Gene Ther. 2021. PMID: 34533444 Review.
-
Placental mesenchymal stromal cells as an alternative tool for therapeutic angiogenesis.Cell Mol Life Sci. 2020 Jan;77(2):253-265. doi: 10.1007/s00018-019-03268-1. Epub 2019 Aug 29. Cell Mol Life Sci. 2020. PMID: 31468060 Free PMC article. Review.
Cited by
-
Renal subcapsular delivery of PGE2 promotes kidney repair by activating endogenous Sox9+ stem cells.iScience. 2021 Oct 8;24(11):103243. doi: 10.1016/j.isci.2021.103243. eCollection 2021 Nov 19. iScience. 2021. PMID: 34746706 Free PMC article.
-
Comparison of the Biological Characteristics of Mesenchymal Stem Cells Derived from the Human Placenta and Umbilical Cord.Sci Rep. 2018 Mar 22;8(1):5014. doi: 10.1038/s41598-018-23396-1. Sci Rep. 2018. PMID: 29568084 Free PMC article.
-
Conditioned medium from primary cytotrophoblasts, primary placenta-derived mesenchymal stem cells, or sub-cultured placental tissue promoted HUVEC angiogenesis in vitro.Stem Cell Res Ther. 2021 Feb 17;12(1):141. doi: 10.1186/s13287-021-02192-1. Stem Cell Res Ther. 2021. PMID: 33596987 Free PMC article.
-
Human pancreatic islet-derived stromal cells reveal combined features of mesenchymal stromal cells and pancreatic stellate cells.Stem Cell Res Ther. 2024 Oct 8;15(1):351. doi: 10.1186/s13287-024-03963-2. Stem Cell Res Ther. 2024. PMID: 39380125 Free PMC article.
-
Heterogeneity of mesenchymal stem cells in cartilage regeneration: from characterization to application.NPJ Regen Med. 2021 Mar 19;6(1):14. doi: 10.1038/s41536-021-00122-6. NPJ Regen Med. 2021. PMID: 33741999 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

