Linking Murine and Human Plasmodium falciparum Challenge Models in a Translational Path for Antimalarial Drug Development
- PMID: 27044554
- PMCID: PMC4879391
- DOI: 10.1128/AAC.02883-15
Linking Murine and Human Plasmodium falciparum Challenge Models in a Translational Path for Antimalarial Drug Development
Abstract
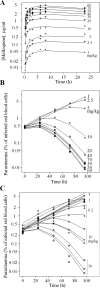
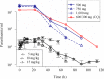
Effective progression of candidate antimalarials is dependent on optimal dosing in clinical studies, which is determined by a sound understanding of pharmacokinetics and pharmacodynamics (PK/PD). Recently, two important translational models for antimalarials have been developed: the NOD/SCID/IL2Rγ(-/-) (NSG) model, whereby mice are engrafted with noninfected and Plasmodium falciparum-infected human erythrocytes, and the induced blood-stage malaria (IBSM) model in human volunteers. The antimalarial mefloquine was used to directly measure the PK/PD in both models, which were compared to previously published trial data for malaria patients. The clinical part was a single-center, controlled study using a blood-stage Plasmodium falciparum challenge inoculum in volunteers to characterize the effectiveness of mefloquine against early malaria. The study was conducted in three cohorts (n = 8 each) using different doses of mefloquine. The characteristic delay in onset of action of about 24 h was seen in both NSG and IBSM systems. In vivo 50% inhibitory concentrations (IC50s) were estimated at 2.0 μg/ml and 1.8 μg/ml in the NSG and IBSM models, respectively, aligning with 1.8 μg/ml reported previously for patients. In the IBSM model, the parasite reduction ratios were 157 and 195 for the 10- and 15-mg/kg doses, within the range of previously reported clinical data for patients but significantly lower than observed in the mouse model. Linking mouse and human challenge models to clinical trial data can accelerate the accrual of critical data on antimalarial drug activity. Such data can guide large clinical trials required for development of urgently needed novel antimalarial combinations. (This trial was registered at the Australian New Zealand Clinical Trials Registry [http://anzctr.org.au] under registration number ACTRN12612000323820.).
Copyright © 2016 McCarthy et al.
Figures

References
-
- Craig AG, Grau GE, Janse C, Kazura JW, Milner D, Barnwell JW, Turner G, Langhorne J, participants of the Hinxton Retreat Meeting on Animal Models for Research on Severe Malaria . 2012. The role of animal models for research on severe malaria. PLoS Pathog 8:e1002401. doi:10.1371/journal.ppat.1002401. - DOI - PMC - PubMed
-
- Sanderson F, Andrews L, Douglas AD, Hunt-Cooke A, Bejon P, Hill AV. 2008. Blood-stage challenge for malaria vaccine efficacy trials: a pilot study with discussion of safety and potential value. Am J Trop Med Hyg 78:878–883. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

