The Carbohydrate-linked Phosphorylcholine of the Parasitic Nematode Product ES-62 Modulates Complement Activation
- PMID: 27044740
- PMCID: PMC4882459
- DOI: 10.1074/jbc.M115.702746
The Carbohydrate-linked Phosphorylcholine of the Parasitic Nematode Product ES-62 Modulates Complement Activation
Abstract
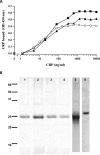
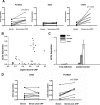
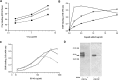
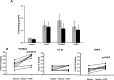
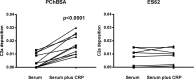
Parasitic nematodes manufacture various carbohydrate-linked phosphorylcholine (PCh)-containing molecules, including ES-62, a protein with an N-linked glycan terminally substituted with PCh. The PCh component is biologically important because it is required for immunomodulatory effects. We showed that most ES-62 was bound to a single protein, C-reactive protein (CRP), in normal human serum, displaying a calcium-dependent, high-avidity interaction and ability to form large complexes. Unexpectedly, CRP binding to ES-62 failed to efficiently activate complement as far as the C3 convertase stage in comparison with PCh-BSA and PCh-containing Streptococcus pneumoniae cell wall polysaccharide. C1q capture assays demonstrated an ES-62-CRP-C1q interaction in serum. The three ligands all activated C1 and generated C4b to similar extents. However, a C2a active site was not generated following ES-62 binding to CRP, demonstrating that C2 cleavage was far less efficient for ES-62-containing complexes. We proposed that failure of C2 cleavage was due to the flexible nature of carbohydrate-bound PCh and that reduced proximity of the C1 complex was the reason that C2 was poorly cleaved. This was confirmed using synthetic analogues that were similar to ES-62 only in respect of having a flexible PCh. Furthermore, ES-62 was shown to deplete early complement components, such as the rate-limiting C4, following CRP interaction and thereby inhibit classical pathway activation. Thus, flexible PCh-glycan represents a novel mechanism for subversion of complement activation. These data illustrate the importance of the rate-limiting C4/C2 stage of complement activation and reveal a new addition to the repertoire of ES-62 immunomodulatory mechanisms with possible therapeutic applications.
Keywords: C-reactive protein; C1Q complex (C1QA); ES-62; acute phase reactant; carbohydrate function; complement; immunomodulation; parasite; pentraxin; phosphorylcholine.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



References
-
- Maizels R. M., Burke J., and Denham D. A. (1987) Phosphorylcholine bearing antigens in filarial nematode parasites: analysis of somatic extracts, in vitro secretions and infection sera from B. malayi and B. pahangi. Parasite Immunol. 9, 49–66 - PubMed
-
- Hewitson J. P., Harcus Y. M., Curwen R. S., Dowle A. A., Atmadja A. K., Ashton P. D., Wilson A., and Maizels R. M. (2008) The secretome of the filarial parasite, Brugia malayi: proteomic profile of adult excretory-secretory products. Mol. Biochem. Parasitol. 160, 8–21 - PubMed
-
- Lal R. B., and Ottesen E. A. (1989) Phosphocholine epitopes on helminth and protozoal parasites and their presence in the circulation of infected human patients. Trans. R Soc. Trop. Med. Hyg. 83, 652–655 - PubMed
-
- Haslam S. M., Houston K. M., Harnett W., Reason A. J., and Morris H. R., and Dell A. (1999) Structural studies of N glycans of filarial parasite: conservation of phosphorylcholine substituted glycans among species and discovery of novel chito-oligomers. J. Biol. Chem. 274, 20953–20960 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

