Structural Analysis of the Myo1c and Neph1 Complex Provides Insight into the Intracellular Movement of Neph1
- PMID: 27044863
- PMCID: PMC4959310
- DOI: 10.1128/MCB.00020-16
Structural Analysis of the Myo1c and Neph1 Complex Provides Insight into the Intracellular Movement of Neph1
Abstract
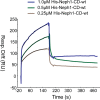
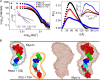
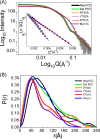
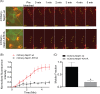
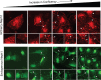
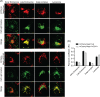
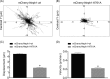
The Myo1c motor functions as a cargo transporter supporting various cellular events, including vesicular trafficking, cell migration, and stereociliary movements of hair cells. Although its partial crystal structures were recently described, the structural details of its interaction with cargo proteins remain unknown. This study presents the first structural demonstration of a cargo protein, Neph1, attached to Myo1c, providing novel insights into the role of Myo1c in intracellular movements of this critical slit diaphragm protein. Using small angle X-ray scattering studies, models of predominant solution conformation of unliganded full-length Myo1c and Myo1c bound to Neph1 were constructed. The resulting structures show an extended S-shaped Myo1c with Neph1 attached to its C-terminal tail. Importantly, binding of Neph1 did not induce a significant shape change in Myo1c, indicating this as a spontaneous process or event. Analysis of interaction surfaces led to the identification of a critical residue in Neph1 involved in binding to Myo1c. Indeed, a point mutant from this site abolished interaction between Neph1 and Myo1c when tested in the in vitro and in live-cell binding assays. Live-cell imaging, including fluorescence recovery after photobleaching, provided further support for the role of Myo1c in intracellular vesicular movement of Neph1 and its turnover at the membrane.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures




References
-
- Welsh GI, Saleem MA. 2012. The podocyte cytoskeleton—key to a functioning glomerulus in health and disease. Nat Rev Nephrol 8:14–21. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
