Endothelial Mitogen-Activated Protein Kinase Kinase Kinase Kinase 4 Is Critical for Lymphatic Vascular Development and Function
- PMID: 27044870
- PMCID: PMC4907094
- DOI: 10.1128/MCB.01121-15
Endothelial Mitogen-Activated Protein Kinase Kinase Kinase Kinase 4 Is Critical for Lymphatic Vascular Development and Function
Abstract
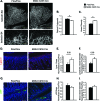
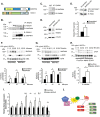
The molecular mechanisms underlying lymphatic vascular development and function are not well understood. Recent studies have suggested a role for endothelial cell (EC) mitogen-activated protein kinase kinase kinase kinase 4 (Map4k4) in developmental angiogenesis and atherosclerosis. Here, we show that constitutive loss of EC Map4k4 in mice causes postnatal lethality due to chylothorax, suggesting that Map4k4 is required for normal lymphatic vascular function. Mice constitutively lacking EC Map4k4 displayed dilated lymphatic capillaries, insufficient lymphatic valves, and impaired lymphatic flow; furthermore, primary ECs derived from these animals displayed enhanced proliferation compared with controls. Yeast 2-hybrid analyses identified the Ras GTPase-activating protein Rasa1, a known regulator of lymphatic development and lymphatic endothelial cell fate, as a direct interacting partner for Map4k4. Map4k4 silencing in ECs enhanced basal Ras and extracellular signal-regulated kinase (Erk) activities, and primary ECs lacking Map4k4 displayed enhanced lymphatic EC marker expression. Taken together, these results reveal that EC Map4k4 is critical for lymphatic vascular development by regulating EC quiescence and lymphatic EC fate.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures



Similar articles
-
Map4k4 impairs energy metabolism in endothelial cells and promotes insulin resistance in obesity.Am J Physiol Endocrinol Metab. 2017 Sep 1;313(3):E303-E313. doi: 10.1152/ajpendo.00037.2017. Epub 2017 Jun 13. Am J Physiol Endocrinol Metab. 2017. PMID: 28611026 Free PMC article.
-
MAP4K4 is a novel MAPK/ERK pathway regulator required for lung adenocarcinoma maintenance.Mol Oncol. 2017 Jun;11(6):628-639. doi: 10.1002/1878-0261.12055. Epub 2017 May 2. Mol Oncol. 2017. PMID: 28306189 Free PMC article.
-
Endothelial protein kinase MAP4K4 promotes vascular inflammation and atherosclerosis.Nat Commun. 2015 Dec 21;6:8995. doi: 10.1038/ncomms9995. Nat Commun. 2015. PMID: 26688060 Free PMC article.
-
Map4k4 Signaling Nodes in Metabolic and Cardiovascular Diseases.Trends Endocrinol Metab. 2016 Jul;27(7):484-492. doi: 10.1016/j.tem.2016.04.006. Epub 2016 May 6. Trends Endocrinol Metab. 2016. PMID: 27160798 Free PMC article. Review.
-
EPHB4-RASA1-Mediated Negative Regulation of Ras-MAPK Signaling in the Vasculature: Implications for the Treatment of EPHB4- and RASA1-Related Vascular Anomalies in Humans.Pharmaceuticals (Basel). 2023 Jan 23;16(2):165. doi: 10.3390/ph16020165. Pharmaceuticals (Basel). 2023. PMID: 37259315 Free PMC article. Review.
Cited by
-
A novel role of Hippo-Yap/TAZ signaling pathway in lymphatic vascular development.BMB Rep. 2021 Jun;54(6):285-294. doi: 10.5483/BMBRep.2021.54.6.020. BMB Rep. 2021. PMID: 33691907 Free PMC article. Review.
-
Low Efficacy of Genetic Tests for the Diagnosis of Primary Lymphedema Prompts Novel Insights into the Underlying Molecular Pathways.Int J Mol Sci. 2022 Jul 3;23(13):7414. doi: 10.3390/ijms23137414. Int J Mol Sci. 2022. PMID: 35806420 Free PMC article.
-
Modulation of calcium signaling and metabolic pathways in endothelial cells with magnetic fields.Nanoscale Adv. 2024 Jan 23;6(4):1163-1182. doi: 10.1039/d3na01065a. eCollection 2024 Feb 13. Nanoscale Adv. 2024. PMID: 38356636 Free PMC article.
-
Role of RASA1 in cancer: A review and update (Review).Oncol Rep. 2020 Dec;44(6):2386-2396. doi: 10.3892/or.2020.7807. Epub 2020 Oct 13. Oncol Rep. 2020. PMID: 33125148 Free PMC article. Review.
-
Abrogation of MAP4K4 protein function causes congenital anomalies in humans and zebrafish.Sci Adv. 2023 Apr 28;9(17):eade0631. doi: 10.1126/sciadv.ade0631. Epub 2023 Apr 26. Sci Adv. 2023. PMID: 37126546 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
