Kindlin-2 directly binds actin and regulates integrin outside-in signaling
- PMID: 27044892
- PMCID: PMC4828686
- DOI: 10.1083/jcb.201501006
Kindlin-2 directly binds actin and regulates integrin outside-in signaling
Abstract
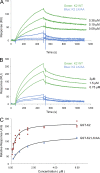
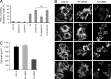
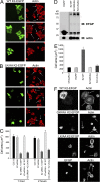
Reduced levels of kindlin-2 (K2) in endothelial cells derived from K2(+/-)mice or C2C12 myoblastoid cells treated with K2 siRNA showed disorganization of their actin cytoskeleton and decreased spreading. These marked changes led us to examine direct binding between K2 and actin. Purified K2 interacts with F-actin in cosedimentation and surface plasmon resonance analyses and induces actin aggregation. We further find that the F0 domain of K2 binds actin. A mutation, LK(47)/AA, within a predicted actin binding site (ABS) of F0 diminishes its interaction with actin by approximately fivefold. Wild-type K2 and K2 bearing the LK(47)/AA mutation were equivalent in their ability to coactivate integrin αIIbβ3 in a CHO cell system when coexpressed with talin. However, K2-LK(47)/AA exhibited a diminished ability to support cell spreading and actin organization compared with wild-type K2. The presence of an ABS in F0 of K2 that influences outside-in signaling across integrins establishes a new foundation for considering how kindlins might regulate cellular responses.
© 2016 Bledzka et al.
Figures



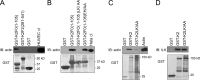


References
-
- Bialkowska K., Ma Y.Q., Bledzka K., Sossey-Alaoui K., Izem L., Zhang X., Malinin N., Qin J., Byzova T., and Plow E.F.. 2010. The integrin co-activator Kindlin-3 is expressed and functional in a non-hematopoietic cell, the endothelial cell. J. Biol. Chem. 285:18640–18649. 10.1074/jbc.M109.085746 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

