Afatinib Activity in Platinum-Refractory Metastatic Urothelial Carcinoma in Patients With ERBB Alterations
- PMID: 27044931
- PMCID: PMC5569685
- DOI: 10.1200/JCO.2015.66.3047
Afatinib Activity in Platinum-Refractory Metastatic Urothelial Carcinoma in Patients With ERBB Alterations
Erratum in
-
Erratum.J Clin Oncol. 2017 Feb;35(4):478. doi: 10.1200/JCO.2016.72.0888. J Clin Oncol. 2017. PMID: 28129521 Free PMC article. No abstract available.
Abstract
Purpose: Somatic mutations and copy number variation in the ERBB family are frequent in urothelial carcinoma (UC) and may represent viable therapeutic targets. We studied whether afatinib (an oral, irreversible inhibitor of the ErbB family) has activity in UC and if specific ERBB molecular alterations are associated with clinical response.
Patients and methods: In this phase II trial, patients with metastatic platinum-refractory UC received afatinib 40 mg/day continuously until progression or intolerance. The primary end point was 3-month progression-free survival (PFS3). Prespecified tumor analysis for alterations in EGFR, HER2, ERBB3, and ERBB4 was conducted.
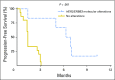
Results: The first-stage enrollment goal of 23 patients was met. Patient demographic data included: 78% male, median age 67 years (range, 36 to 82 years), hemoglobin < 10 g/dL in 17%, liver metastases in 30%, median time from prior chemotherapy of 3.6 months, and Eastern Cooperative Oncology Group performance status ≤ 1 in 100%. No unexpected toxicities were observed; two patients required dose reduction for grade 3 fatigue and rash. Overall, five of 23 patients (21.7%) met PFS3 (two partial response, three stable disease). Notably, among the 21 tumors analyzed, five of six patients (83.3%) with HER2 and/or ERBB3 alterations achieved PFS3 (PFS = 10.3, 7.0, 6.9, 6.3, and 5.0 months, respectively) versus none of 15 patients without alterations (P < .001). Three of four patients with HER2 amplification and three of three patients with ERBB3 somatic mutations (G284R, V104M, and R103G) met PFS3. One patient with both HER2 amplification and ERBB3 mutation never progressed on therapy, but treatment was discontinued after 10.3 months as a result of depressed ejection fraction. The median time to progression/discontinuation was 6.6 months in patients with HER2/ERBB3 alterations versus 1.4 months in patients without alterations (P < .001).
Conclusion: Afatinib demonstrated significant activity in patients with platinum-refractory UC with HER2 or ERBB3 alterations. The potential contribution of ERBB3 to afatinib sensitivity is novel. Afatinib deserves further investigation in molecularly selected UC.
© 2016 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest are found in the article online at
Figures



References
-
- Society AC. Cancer Facts and Figures 2015. Atlanta, GA: American Cancer Society; 2015.
-
- Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558–562. - PubMed
-
- Chow NH, Chan SH, Tzai TS, et al. Expression profiles of ErbB family receptors and prognosis in primary transitional cell carcinoma of the urinary bladder. Clin Cancer Res. 2001;7:1957–1962. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

