Vaccination of Gerbils with Bm-103 and Bm-RAL-2 Concurrently or as a Fusion Protein Confers Consistent and Improved Protection against Brugia malayi Infection
- PMID: 27045170
- PMCID: PMC4821550
- DOI: 10.1371/journal.pntd.0004586
Vaccination of Gerbils with Bm-103 and Bm-RAL-2 Concurrently or as a Fusion Protein Confers Consistent and Improved Protection against Brugia malayi Infection
Abstract
Background: The Brugia malayi Bm-103 and Bm-RAL-2 proteins are orthologous to Onchocerca volvulus Ov-103 and Ov-RAL-2, and which were selected as the best candidates for the development of an O. volvulus vaccine. The B. malayi gerbil model was used to confirm the efficacy of these Ov vaccine candidates on adult worms and to determine whether their combination is more efficacious.
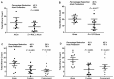
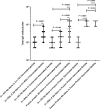
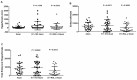
Methodology and principle findings: Vaccine efficacy of recombinant Bm-103 and Bm-RAL-2 administered individually, concurrently or as a fusion protein were tested in gerbils using alum as adjuvant. Vaccination with Bm-103 resulted in worm reductions of 39%, 34% and 22% on 42, 120 and 150 days post infection (dpi), respectively, and vaccination with Bm-RAL-2 resulted in worm reductions of 42%, 22% and 46% on 42, 120 and 150 dpi, respectively. Vaccination with a fusion protein comprised of Bm-103 and Bm-RAL-2 resulted in improved efficacy with significant reduction of worm burden of 51% and 49% at 90 dpi, as did the concurrent vaccination with Bm-103 and Bm-RAL-2, with worm reduction of 61% and 56% at 90 dpi. Vaccination with Bm-103 and Bm-RAL-2 as a fusion protein or concurrently not only induced a significant worm reduction of 61% and 42%, respectively, at 150 dpi, but also significantly reduced the fecundity of female worms as determined by embryograms. Elevated levels of antigen-specific IgG were observed in all vaccinated gerbils. Serum from gerbils vaccinated with Bm-103 and Bm-RAL-2 individually, concurrently or as a fusion protein killed third stage larvae in vitro when combined with peritoneal exudate cells.
Conclusion: Although vaccination with Bm-103 and Bm-RAL-2 individually conferred protection against B. malayi infection in gerbils, a more consistent and enhanced protection was induced by vaccination with Bm-103 and Bm-RAL-2 fusion protein and when they were used concurrently. Further characterization and optimization of these filarial vaccines are warranted.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Ligand binding properties of two Brugia malayi fatty acid and retinol (FAR) binding proteins and their vaccine efficacies against challenge infection in gerbils.PLoS Negl Trop Dis. 2018 Oct 8;12(10):e0006772. doi: 10.1371/journal.pntd.0006772. eCollection 2018 Oct. PLoS Negl Trop Dis. 2018. PMID: 30296268 Free PMC article.
-
Vaccination with recombinant Brugia malayi cystatin proteins alters worm migration, homing and final niche selection following a subcutaneous challenge of Mongolian gerbils (Meriones unguiculatus) with B. malayi infective larvae.Parasit Vectors. 2014 Jan 22;7:43. doi: 10.1186/1756-3305-7-43. Parasit Vectors. 2014. PMID: 24450869 Free PMC article.
-
DNA vaccine encoding the moonlighting protein Onchocerca volvulus glyceraldehyde-3-phosphate dehydrogenase (Ov-GAPDH) leads to partial protection in a mouse model of human filariasis.Vaccine. 2015 Oct 26;33(43):5861-5867. doi: 10.1016/j.vaccine.2015.07.110. Epub 2015 Aug 29. Vaccine. 2015. PMID: 26320419
-
Immune evasion genes from filarial nematodes.Int J Parasitol. 2001 Jul;31(9):889-98. doi: 10.1016/s0020-7519(01)00213-2. Int J Parasitol. 2001. PMID: 11406138 Review.
-
Future prospects and challenges of vaccines against filariasis.Parasite Immunol. 2012 May;34(5):243-53. doi: 10.1111/j.1365-3024.2011.01350.x. Parasite Immunol. 2012. PMID: 22150082 Review.
Cited by
-
Onchocerca volvulus bivalent subunit vaccine induces protective immunity in genetically diverse collaborative cross recombinant inbred intercross mice.NPJ Vaccines. 2021 Jan 26;6(1):17. doi: 10.1038/s41541-020-00276-2. NPJ Vaccines. 2021. PMID: 33500417 Free PMC article.
-
Biosynthesis and characterization of Ocimum sanctum green silver nanoparticles and unravelling their enhanced anti-filarial activity through a HRAMS proteomics approach.RSC Adv. 2024 Feb 14;14(9):5893-5906. doi: 10.1039/d3ra08702f. eCollection 2024 Feb 14. RSC Adv. 2024. PMID: 38362069 Free PMC article.
-
Stage-Specific Transcriptome and Proteome Analyses of the Filarial Parasite Onchocerca volvulus and Its Wolbachia Endosymbiont.mBio. 2016 Nov 23;7(6):e02028-16. doi: 10.1128/mBio.02028-16. mBio. 2016. PMID: 27881553 Free PMC article.
-
Computational and experimental analysis of the glycophosphatidylinositol-anchored proteome of the human parasitic nematode Brugia malayi.PLoS One. 2019 Sep 12;14(9):e0216849. doi: 10.1371/journal.pone.0216849. eCollection 2019. PLoS One. 2019. PMID: 31513600 Free PMC article.
-
Onchocerca volvulus: The Road from Basic Biology to a Vaccine.Trends Parasitol. 2018 Jan;34(1):64-79. doi: 10.1016/j.pt.2017.08.011. Epub 2017 Sep 22. Trends Parasitol. 2018. PMID: 28958602 Free PMC article. Review.
References
-
- Vos T, Barber RM, Bell B, Bertozzi-Villa A, Biryukov S, Bolliger I, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015; 386(9995): 743–800. 10.1016/S0140-6736(15)60692-4 - DOI - PMC - PubMed
-
- Eng JK, Prichard RK. A comparison of genetic polymorphism in populations of Onchocerca volvulus from untreated- and ivermectin-treated patients. Mol Biochem Parasitol. 2005; 142: 193–202. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

