Ultrasensitive microfluidic analysis of circulating exosomes using a nanostructured graphene oxide/polydopamine coating
- PMID: 27045543
- PMCID: PMC4970962
- DOI: 10.1039/c6lc00279j
Ultrasensitive microfluidic analysis of circulating exosomes using a nanostructured graphene oxide/polydopamine coating
Abstract
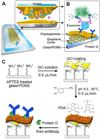
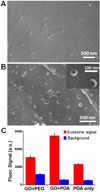
Exosomes are cell-derived nano-sized vesicles that have been recently recognized as new mediators for many cellular processes and potential biomarkers for non-invasive disease diagnosis and the monitoring of treatment response. To better elucidate the biology and clinical value of exosomes, there is a pressing need for new analytical technologies capable of the efficient isolation and sensitive analysis of such small and molecularly diverse vesicles. Herein, we developed a microfluidic exosome analysis platform based on a new graphene oxide/polydopamine (GO/PDA) nano-interface. To the best of our best knowledge, we report for the first time, the GO-induced formation of a 3D nanoporous PDA surface coating enabled by the microfluidic layer-by-layer deposition of GO and PDA. It was demonstrated that this nanostructured GO/PDA interface greatly improves the efficiency of exosome immuno-capture, while at the same time effectively suppressing non-specific exosome adsorption. Based on this nano-interface, an ultrasensitive exosome ELISA assay was developed to afford a very low detection limit of 50 μL(-1) with a 4 log dynamic range, which is substantially better than the existing methods. As a proof of concept for clinical applications, we adapted this platform to discriminate ovarian cancer patients from healthy controls by the quantitative detection of exosomes directly from 2 μL plasma without sample processing. Thus, this platform could provide a useful tool to facilitate basic and clinical investigations of exosomes for non-invasive disease diagnosis and to aid precision treatment.
Figures



Similar articles
-
Facile preparation of protein stationary phase based on polydopamine/graphene oxide platform for chip-based open tubular capillary electrochromatography enantioseparation.J Chromatogr A. 2014 Jan 3;1323:135-42. doi: 10.1016/j.chroma.2013.11.048. Epub 2013 Dec 1. J Chromatogr A. 2014. PMID: 24331371
-
Polydopamine-assisted aptamer-carrying tetrahedral DNA microelectrode sensor for ultrasensitive electrochemical detection of exosomes.J Nanobiotechnology. 2024 Feb 8;22(1):55. doi: 10.1186/s12951-024-02318-6. J Nanobiotechnology. 2024. PMID: 38331774 Free PMC article.
-
A Planar-Gate Graphene Field-Effect Transistor Integrated Portable Platform for Rapid Detection of Colon Cancer-Derived Exosomes.Biosensors (Basel). 2025 Mar 24;15(4):207. doi: 10.3390/bios15040207. Biosensors (Basel). 2025. PMID: 40277521 Free PMC article.
-
Exosome isolation using nanostructures and microfluidic devices.Biomed Mater. 2021 Feb 17;16(2):022005. doi: 10.1088/1748-605X/abde70. Biomed Mater. 2021. PMID: 33477118 Free PMC article. Review.
-
Cancer Liquid Biopsy Using Integrated Microfluidic Exosome Analysis Platforms.Biotechnol J. 2020 May;15(5):e1900225. doi: 10.1002/biot.201900225. Epub 2020 Feb 20. Biotechnol J. 2020. PMID: 32032977 Review.
Cited by
-
Electrochemical Genosensing of Overexpressed GAPDH Transcripts in Breast Cancer Exosomes.Anal Chem. 2023 Jan 31;95(4):2487-2495. doi: 10.1021/acs.analchem.2c04773. Epub 2023 Jan 22. Anal Chem. 2023. PMID: 36683335 Free PMC article.
-
Recent Advances in Device Engineering and Computational Analysis for Characterization of Cell-Released Cancer Biomarkers.Cancers (Basel). 2022 Jan 7;14(2):288. doi: 10.3390/cancers14020288. Cancers (Basel). 2022. PMID: 35053452 Free PMC article. Review.
-
Nanomaterials assisted exosomes isolation and analysis towards liquid biopsy.Mater Today Bio. 2022 Jul 22;16:100371. doi: 10.1016/j.mtbio.2022.100371. eCollection 2022 Dec. Mater Today Bio. 2022. PMID: 35937576 Free PMC article.
-
An ultrasensitive polydopamine bi-functionalized SERS immunoassay for exosome-based diagnosis and classification of pancreatic cancer.Chem Sci. 2018 May 23;9(24):5372-5382. doi: 10.1039/c8sc01611a. eCollection 2018 Jun 28. Chem Sci. 2018. PMID: 30009009 Free PMC article.
-
Graphene Oxide-Based Biosensors for Liquid Biopsies in Cancer Diagnosis.Nanomaterials (Basel). 2019 Dec 3;9(12):1725. doi: 10.3390/nano9121725. Nanomaterials (Basel). 2019. PMID: 31816919 Free PMC article. Review.
References
-
- Huber V, Fais S, Iero M, Lugini L, Canese P, Squarcina P, Zaccheddu A, Colone M, Arancia G, Gentile M, Seregni E, Valenti R, Ballabio G, Belli F, Leo E, Parmiani G, Rivoltini L. Gastroenterology. 2005;128:1796–1804. - PubMed
-
- Tauro BJ, Greening DW, Mathias RA, Ji H, Mathivanan S, Scott AM, Simpson RJ. Methods. 2012;56:293–304. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

