Regulation of prefrontal cortex myelination by the microbiota
- PMID: 27045844
- PMCID: PMC4872400
- DOI: 10.1038/tp.2016.42
Regulation of prefrontal cortex myelination by the microbiota
Abstract
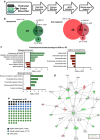
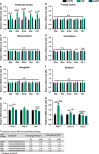
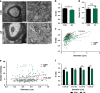
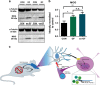
The prefrontal cortex (PFC) is a key region implicated in a range of neuropsychiatric disorders such as depression, schizophrenia and autism. In parallel, the role of the gut microbiota in contributing to these disorders is emerging. Germ-free (GF) animals, microbiota-deficient throughout life, have been instrumental in elucidating the role of the microbiota in many aspects of physiology, especially the role of the microbiota in anxiety-related behaviours, impaired social cognition and stress responsivity. Here we aim to further elucidate the mechanisms of the microbial influence by investigating changes in the homeostatic regulation of neuronal transcription of GF mice within the PFC using a genome-wide transcriptome profiling approach. Our results reveal a marked, concerted upregulation of genes linked to myelination and myelin plasticity. This coincided with upregulation of neural activity-induced pathways, potentially driving myelin plasticity. Subsequent investigation at the ultrastructural level demonstrated the presence of hypermyelinated axons within the PFC of GF mice. Notably, these changes in myelin and activity-related gene expression could be reversed by colonization with a conventional microbiota following weaning. In summary, we believe we demonstrate for the first time that the microbiome is necessary for appropriate and dynamic regulation of myelin-related genes with clear implications for cortical myelination at an ultrastructural level. The microbiota is therefore a potential therapeutic target for psychiatric disorders involving dynamic myelination in the PFC.
Figures
References
-
- Morgan MA, Romanski LM, LeDoux JE. Extinction of emotional learning: contribution of medial prefrontal cortex. Neurosci Lett 1993; 163: 109–113. - PubMed
-
- Sullivan RM, Gratton A. Prefrontal cortical regulation of hypothalamic-pituitary-adrenal function in the rat and implications for psychopathology: side matters. Psychoneuroendocrinology 2002; 27: 99–114. - PubMed
-
- Davidson RJ. Anxiety and affective style: role of prefrontal cortex and amygdala. Biol Psychiatry 2002; 51: 68–80. - PubMed
-
- Goto Y, Yang CR, Otani S. Functional and dysfunctional synaptic plasticity in prefrontal cortex: roles in psychiatric disorders. Biol Psychiatry 2010; 67: 199–207. - PubMed
-
- Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends Neurosci 2008; 31: 137–145. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

