Catalytic, Diastereoselective 1,2-Difluorination of Alkenes
- PMID: 27046019
- PMCID: PMC5097459
- DOI: 10.1021/jacs.6b02391
Catalytic, Diastereoselective 1,2-Difluorination of Alkenes
Abstract
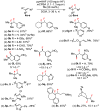
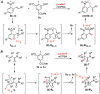
We describe a direct, catalytic approach to the 1,2-difluorination of alkenes. The method utilizes a nucleophilic fluoride source and an oxidant in conjunction with an aryl iodide catalyst and is applicable to alkenes with all types of substitution patterns. In general, the vicinal difluoride products are produced with high diastereoselectivities. The observed sense of stereoinduction implicates anchimeric assistance pathways in reactions of alkenes bearing neighboring Lewis basic functionality.
Figures




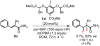
References
-
- Carey FA, Sundberg RJ. Advanced Organic Chemistry Part B: Re actions and Synthesis. 5. Springer; New York: 2007. pp. 298–305.
-
-
For a recent review on catalytic, stereoselective dihalogenation, see Cresswell AJ, Eey STC, Denmark SE. Angew Chem, Int Ed. 2015;54:15642–15682.
-
-
-
For an indirect method method, see Olah GA, Welch JT, Vankar YD, Nojima M, Kerekes I, Olah JA. J Org Chem. 1979;44:3872–3881.
-
-
- Barton DHR, Lister-James J, Hesse RH, Pechet MM, Rozen S. J Chem Soc, Perkin Trans. 1982;1:1105–1110.
- Purrington ST, Kagen BS, Patrick TB. Chem Rev. 1986;86:997–1018.
- Sanford G. J Fluorine Chem. 2007;128:90–104.
-
- Tius MA. Tetrahedron. 1995;51:6605–6634.
- Tamura M, Takagi T, Quan HD, Sekiya A. J Fluorine Chem. 1999;98:163–166.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

