Structure-guided design of small-molecule therapeutics against RSV disease
- PMID: 27046051
- PMCID: PMC5074927
- DOI: 10.1517/17460441.2016.1174212
Structure-guided design of small-molecule therapeutics against RSV disease
Abstract
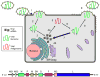
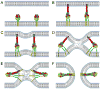
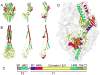
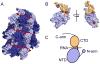
In the United States, respiratory syncytial virus (RSV) is responsible for the majority of infant hospitalizations resulting from viral infections, as well as a leading source of pneumonia and bronchiolitis in young children and the elderly. In the absence of vaccine prophylaxis or an effective antiviral for improved disease management, the development of novel anti-RSV therapeutics is critical. Several advanced drug development campaigns of the past decade have focused on blocking viral infection. These efforts have returned a chemically distinct panel of small-molecule RSV entry inhibitors, but binding sites and molecular mechanism of action appeared to share a common mechanism, resulting in comprehensive cross-resistance and calling for alternative druggable targets such as viral RNA-dependent RNA-polymerase complex. Areas Covered: In this review, the authors discuss the current status of the mechanism of action of RSV entry inhibitors. They also provide the recent structural insight into the organization of the polymerase complex that have revealed novel drug targets sites, and outline a path towards the discovery of next-generation RSV therapeutics. Expert opinion: Considering the tremendous progress experienced in our structural understanding of RSV biology in recent years and encouraging early results of a nucleoside analog inhibitor in clinical trials, there is high prospect that new generations of much needed effective anti-RSV therapeutics will become available for clinical use in the foreseeable future.
Keywords: RNA-dependent RNA-polymerase; Respiratory syncytial virus; small molecule antiviral; viral replication; virus entry.
Figures

Similar articles
-
Cross-resistance mechanism of respiratory syncytial virus against structurally diverse entry inhibitors.Proc Natl Acad Sci U S A. 2014 Aug 19;111(33):E3441-9. doi: 10.1073/pnas.1405198111. Epub 2014 Aug 4. Proc Natl Acad Sci U S A. 2014. PMID: 25092342 Free PMC article.
-
The paramyxovirus polymerase complex as a target for next-generation anti-paramyxovirus therapeutics.Front Microbiol. 2015 May 12;6:459. doi: 10.3389/fmicb.2015.00459. eCollection 2015. Front Microbiol. 2015. PMID: 26029193 Free PMC article. Review.
-
Mechanism of Cross-Resistance to Fusion Inhibitors Conferred by the K394R Mutation in Respiratory Syncytial Virus Fusion Protein.J Virol. 2021 Sep 27;95(20):e0120521. doi: 10.1128/JVI.01205-21. Epub 2021 Aug 11. J Virol. 2021. PMID: 34379500 Free PMC article.
-
Inhibitors of respiratory syncytial virus replication target cotranscriptional mRNA guanylylation by viral RNA-dependent RNA polymerase.J Virol. 2005 Oct;79(20):13105-15. doi: 10.1128/JVI.79.20.13105-13115.2005. J Virol. 2005. PMID: 16189012 Free PMC article.
-
Emerging small and large molecule therapeutics for respiratory syncytial virus.Expert Opin Investig Drugs. 2020 Mar;29(3):285-294. doi: 10.1080/13543784.2020.1735349. Epub 2020 Feb 27. Expert Opin Investig Drugs. 2020. PMID: 32096420 Review.
Cited by
-
RSV hijacks cellular protein phosphatase 1 to regulate M2-1 phosphorylation and viral transcription.PLoS Pathog. 2018 Feb 28;14(3):e1006920. doi: 10.1371/journal.ppat.1006920. eCollection 2018 Mar. PLoS Pathog. 2018. PMID: 29489893 Free PMC article.
-
Identification and Characterization of Influenza Virus Entry Inhibitors through Dual Myxovirus High-Throughput Screening.J Virol. 2016 Jul 27;90(16):7368-7387. doi: 10.1128/JVI.00898-16. Print 2016 Aug 15. J Virol. 2016. PMID: 27252534 Free PMC article.
-
Targeting the Respiratory Syncytial Virus N0-P Complex with Constrained α-Helical Peptides in Cells and Mice.Antimicrob Agents Chemother. 2020 Sep 21;64(10):e00717-20. doi: 10.1128/AAC.00717-20. Print 2020 Sep 21. Antimicrob Agents Chemother. 2020. PMID: 32660994 Free PMC article.
-
Evaluation of Small Molecule Combinations against Respiratory Syncytial Virus In Vitro.Molecules. 2021 Apr 29;26(9):2607. doi: 10.3390/molecules26092607. Molecules. 2021. PMID: 33946996 Free PMC article.
-
Self-capping of nucleoprotein filaments protects the Newcastle disease virus genome.Elife. 2019 Jul 10;8:e45057. doi: 10.7554/eLife.45057. Elife. 2019. PMID: 31290740 Free PMC article.
References
-
- Collins PLCJ., Jr . Respiratory Syncytial Virus and Metapneumoviruses. In: Knipe DMHP, editor. Fields Virology. Lippincott, Williams, & Wilkins; Philadelphia: 2007. pp. 1601–1645.
-
- Shay DK, Holman RC, Roosevelt GE, Clarke MJ, Anderson LJ. Bronchiolitis-associated mortality and estimates of respiratory syncytial virus-associated deaths among US children, 1979–1997. J Infect Dis. 2001;183:16–22. - PubMed
-
- Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O’Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA, 3rd, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De Leon FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. - PMC - PubMed
-
- Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O’Brien KL, Roca A, Wright PF, Bruce N, Chandran A, Theodoratou E, Sutanto A, Sedyaningsih ER, Ngama M, Munywoki PK, Kartasasmita C, Simoes EA, Rudan I, Weber MW, Campbell H. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375:1545–1555. - PMC - PubMed
-
- Falsey AR. Respiratory syncytial virus infection in elderly and high-risk adults. Exp Lung Res. 2005;31(Suppl 1):77. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
