Factor XI Deficiency Alters the Cytokine Response and Activation of Contact Proteases during Polymicrobial Sepsis in Mice
- PMID: 27046148
- PMCID: PMC4821616
- DOI: 10.1371/journal.pone.0152968
Factor XI Deficiency Alters the Cytokine Response and Activation of Contact Proteases during Polymicrobial Sepsis in Mice
Abstract
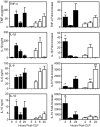
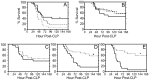
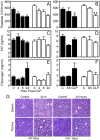
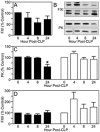
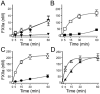
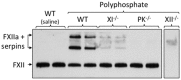
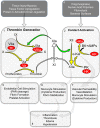
Sepsis, a systemic inflammatory response to infection, is often accompanied by abnormalities of blood coagulation. Prior work with a mouse model of sepsis induced by cecal ligation and puncture (CLP) suggested that the protease factor XIa contributed to disseminated intravascular coagulation (DIC) and to the cytokine response during sepsis. We investigated the importance of factor XI to cytokine and coagulation responses during the first 24 hours after CLP. Compared to wild type littermates, factor XI-deficient (FXI-/-) mice had a survival advantage after CLP, with smaller increases in plasma levels of TNF-α and IL-10 and delayed IL-1β and IL-6 responses. Plasma levels of serum amyloid P, an acute phase protein, were increased in wild type mice 24 hours post-CLP, but not in FXI-/- mice, supporting the impression of a reduced inflammatory response in the absence of factor XI. Surprisingly, there was little evidence of DIC in mice of either genotype. Plasma levels of the contact factors factor XII and prekallikrein were reduced in WT mice after CLP, consistent with induction of contact activation. However, factor XII and PK levels were not reduced in FXI-/- animals, indicating factor XI deficiency blunted contact activation. Intravenous infusion of polyphosphate into WT mice also induced changes in factor XII, but had much less effect in FXI deficient mice. In vitro analysis revealed that factor XIa activates factor XII, and that this reaction is enhanced by polyanions such polyphosphate and nucleic acids. These data suggest that factor XI deficiency confers a survival advantage in the CLP sepsis model by altering the cytokine response to infection and blunting activation of the contact (kallikrein-kinin) system. The findings support the hypothesis that factor XI functions as a bidirectional interface between contact activation and thrombin generation, allowing the two processes to influence each other.
Conflict of interest statement
Figures


References
-
- Degen JL, Bugge TH, Goguen JD. Fibrin and fibrinolysis in infection and host defense. J Thromb Haemost. 2007. July;5 Suppl 1:24–31. - PubMed
-
- Pawlinski R, Mackman N. Tissue factor, coagulation proteases, and protease-activated receptors in endotoxemia and sepsis. Crit Care Med. 2004;32 Suppl 5: S293–297. - PubMed
-
- van der Poll T, Levi M. Crosstalk between inflammation and coagulation: the lessons of sepsis. Curr Vasc Pharmacol. 2012. September;10(5):632–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

