A Randomized Controlled Double Blind Trial of Ciclosporin versus Prednisolone in the Management of Leprosy Patients with New Type 1 Reaction, in Ethiopia
- PMID: 27046330
- PMCID: PMC4821535
- DOI: 10.1371/journal.pntd.0004502
A Randomized Controlled Double Blind Trial of Ciclosporin versus Prednisolone in the Management of Leprosy Patients with New Type 1 Reaction, in Ethiopia
Abstract
Background: Leprosy Type 1 (T1R) reactions are immune-mediated events leading to nerve damage and preventable disability affecting hands, feet and eyes. Type 1 Reactions are treated with oral corticosteroids. There is little evidence on alternative treatments for patients who do not respond to steroids or experience steroid adverse effects. We report the results of a randomized controlled trial testing the efficacy and adverse effect profile of ciclosporin and prednisolone (CnP) in comparison to prednisolone only (P) in patients with new T1R in Ethiopia. Ciclosporin is a potent immunosuppressant. Outcomes were measured using a clinical severity score, recurrence rate, adverse events and quality of life.
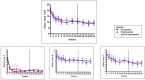
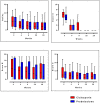
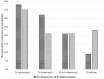
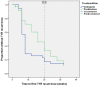
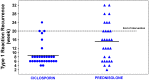
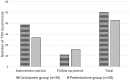
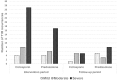
Results: Seventy three patients with new T1R were randomized to receive CnP or P for 20 weeks. Recovery rates in skin signs was similar in both groups (91% vs 88%). Improvements in nerve function both, new and old, sensory (66% vs 49%) and motor (75% vs 74%) loss were higher (but not significantly so) in the patients on CnP. Recurrences rates of T1R (85%) were high in both groups, and recurrences occurred significantly earlier (8 weeks) in patients CnP, who needed 10% more additional prednisolone. Serious major and minor adverse events rates were similar in patients in the two treatment arms of the study. Both groups had a significant improvement in their quality of life after the study, measured by the SF-36.
Conclusions: This is the first double-blind RCT assessing ciclosporin, in the management of T1R in Africa. Ciclosporin could be a safe alternative second-line drug for patients with T1R who are not improving with prednisolone or are experiencing adverse events related to prednisolone. This study illustrates the difficulty in switching off leprosy inflammation. Better treatment agents for leprosy patients with reactions and nerve damage are needed.
Conflict of interest statement
The authors have declared that no competing interests exist. Study drugs and placebo tablets were purchased from Panacea–Biotec (India) and E-Pharm (Ethiopia), both companies with GMP certification.
Figures




References
-
- Lockwood DNJ (2004) Leprosy In: Burns DA, Breathnach SM, Cox NH, Griffiths CEM, editors. Rook’s textbook of Dermatology, 7th ed. Blackwell Publishing, Oxford: pp. 1215–1235.
-
- World Health Organization (2015) Weekly Epidemiological Record. Geneva, Switzerland. 461–476 p.
-
- Croft RP, Nicholls PG, Steyerberg EW, Richardus JH, Cairns W, et al. (2000) A clinical prediction rule for nerve-function impairment in leprosy patients. Lancet 355: 1603–1606. - PubMed
-
- van Brakel WH, Khawas IB (1994) Nerve damage in leprosy: an epidemiological and clinical study of 396 patients in west Nepal—Part 1. Definitions, methods and frequencies. Leprosy Review 65: 204–221. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

