Airway bacteria drive a progressive COPD-like phenotype in mice with polymeric immunoglobulin receptor deficiency
- PMID: 27046438
- PMCID: PMC4822073
- DOI: 10.1038/ncomms11240
Airway bacteria drive a progressive COPD-like phenotype in mice with polymeric immunoglobulin receptor deficiency
Abstract
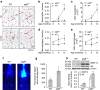
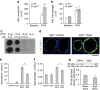
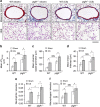
Mechanisms driving persistent airway inflammation in chronic obstructive pulmonary disease (COPD) are incompletely understood. As secretory immunoglobulin A (SIgA) deficiency in small airways has been reported in COPD patients, we hypothesized that immunobarrier dysfunction resulting from reduced SIgA contributes to chronic airway inflammation and disease progression. Here we show that polymeric immunoglobulin receptor-deficient (pIgR(-/-)) mice, which lack SIgA, spontaneously develop COPD-like pathology as they age. Progressive airway wall remodelling and emphysema in pIgR(-/-) mice are associated with an altered lung microbiome, bacterial invasion of the airway epithelium, NF-κB activation, leukocyte infiltration and increased expression of matrix metalloproteinase-12 and neutrophil elastase. Re-derivation of pIgR(-/-) mice in germ-free conditions or treatment with the anti-inflammatory phosphodiesterase-4 inhibitor roflumilast prevents COPD-like lung inflammation and remodelling. These findings show that pIgR/SIgA deficiency in the airways leads to persistent activation of innate immune responses to resident lung microbiota, driving progressive small airway remodelling and emphysema.
Figures




References
-
- Vestbo J. et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am. J. Respir. Crit. Care Med. 187, 347–365 (2013) . - PubMed
-
- Hogg J. C., Macklem P. T. & Thurlbeck W. M. Site and nature of airway obstruction in chronic obstructive lung disease. N. Engl. J. Med. 278, 1355–1360 (1968) . - PubMed
-
- Hogg J. C. et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N. Engl. J. Med. 350, 2645–2653 (2004) . - PubMed
-
- Baraldo S., Turato G. & Saetta M. Pathophysiology of the small airways in chronic obstructive pulmonary disease. Respiration 84, 89–97 (2012) . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL092870/HL/NHLBI NIH HHS/United States
- HL085317/HL/NHLBI NIH HHS/United States
- P40 OD010995/OD/NIH HHS/United States
- R01 HL126176/HL/NHLBI NIH HHS/United States
- L30 HL120265/HL/NHLBI NIH HHS/United States
- P30 DK034987/DK/NIDDK NIH HHS/United States
- 5-P39-DK034987/DK/NIDDK NIH HHS/United States
- U01 CA152662/CA/NCI NIH HHS/United States
- R01 HL105479/HL/NHLBI NIH HHS/United States
- T32 HL094296/HL/NHLBI NIH HHS/United States
- R01 HL085317/HL/NHLBI NIH HHS/United States
- HL126176/HL/NHLBI NIH HHS/United States
- K12 HD043483/HD/NICHD NIH HHS/United States
- HL092870/HL/NHLBI NIH HHS/United States
- UL1 RR024975/RR/NCRR NIH HHS/United States
- HL088263/HL/NHLBI NIH HHS/United States
- HL105479/HL/NHLBI NIH HHS/United States
- R01 HL088263/HL/NHLBI NIH HHS/United States
- 5-P40-OD010995/OD/NIH HHS/United States
- I01 BX002378/BX/BLRD VA/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

