Comparative study on the immunopotentiator effect of ISA 201, ISA 61, ISA 50, ISA 206 used in trivalent foot and mouth disease vaccine
- PMID: 27047016
- PMCID: PMC4774654
- DOI: 10.14202/vetworld.2015.1189-1198
Comparative study on the immunopotentiator effect of ISA 201, ISA 61, ISA 50, ISA 206 used in trivalent foot and mouth disease vaccine
Abstract
Aim: A comparison study was conducted to explore the best internationally available adjuvant that could be used in production of a highly potent foot and mouth disease (FMD) vaccine, that could stimulate a strong immune response and possibly give greater protection against FMD.
Materials and methods: Four experimental batches of trivalent FMD vaccine were prepared with different available oil adjuvants which included Montanide ISA 201, 206, 61 and 50.
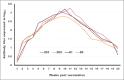
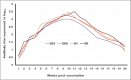
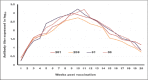
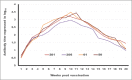
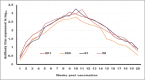
Results: The results indicated that vaccines emulsified using Montanide ISA 201 and Montanide ISA 206 adjuvants elicited a protective humoral immune response from the 2(nd) week postvaccination (WPV) as for ISA 201 with serum neutralization test (SNT) and enzyme-linked immune sorbent assay (ELISA) antibody titers of 1.62±0.047(a) and 1.8±0.049(a), 1.59±0.076(a) and 1.836±0.077(a), and 1.71±0.06(b) and 1.96±0.074(b) for serotypes O, A, SAT2, respectively, and for ISA 206 at SNT and ELISA antibody titers of 1.5±0.082(a) and 1.84±0.084(a), 1.56±0.037(a) and 1.818±0.052(a), and 1.5±0.106(a,b) and 1.81±0.104(a,b) for FMD virus serotypes O, A and SAT2, respectively. For ISA 61 and ISA 50, the protective antibody titer appeared in the 3(rd) WPV. In the ISA 61 FMD vaccine, SNT and ELISA titer were 1.59±0.076(a) and 1.9±0.094(a), 1.53±0.056(a) and 1.83±0.070(a), and 1.5±0.082(a) and 1.84±0.094(a) for serotypes O, A and SAT2, respectively, and in the case of ISA 50 FMD vaccine, the SNT, and ELISA titer were recorded for serotypes O, A and SAT2 respectively, 1.59±0.037(a) and 1.8±0.030(a), 1.68±0.056(a,b) and 1.916±0.065(a,b), and 1.65±0.082(a) and 1.9±0.09(a). On estimating the cellular immune response, the highest delta optical density levels for ISA 201 (0.395-0.460) and ISA 206 (0.375-0.428) were observed on 14 and 21 days post vaccination (DPV) respectively, while the highest levels of lymphoproliferation for ISA 61 (0.375-0.455) and ISA 50 (0.411-0.430) were on 21 and 28 DPV, respectively.
Conclusion: The duration of immunity from Montanide ISA oils (201, 206, 61 and 50) FMD vaccines is a long-lived immunity which ranged between 32 and 38 weeks post vaccination but the Montanide ISA 201 FMD vaccine is superior to the others in the rapid cellular immune response of the vaccinated animals which showed its highest level within 14 days post vaccination.
Keywords: ELISA; FMD Montanide ISA vaccines; SNT; cellular immunity.
Figures


References
-
- Depa P.M., Dimri U., Sharma M.C., Tiwari R. Update on epidemiology and control of foot and mouth disease –A menace to international trade and global animal enterprise. Vet. World. 2012;5(11):694–704.
-
- Rodriguez L.L., Grubman M.J. Foot and mouth disease virus vaccines. Vaccine. 2009;27(Suppl 4):D90–4. - PubMed
-
- Daoud H.M., Ibrahim E.E., El-Din W.M.G., Hassanin A.I.H. Preparation of foot and mouth disease trivalent vaccine type A O, SAT2 and determination of the Guinea pig protective dose 50 (GPPD50) Vet. World. 2013;6(11):844–851.
-
- Ibrahim E.E. Advanced studies on Foot and Mouth Disease Vaccines of sheep in Egypt. Ph.D.Sc. Thesis (Infectious Diseases) Egypt: Faculty of Veterinary Medicine, Cairo University; 2011.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
