The Oscillopathic Nature of Language Deficits in Autism: From Genes to Language Evolution
- PMID: 27047363
- PMCID: PMC4796018
- DOI: 10.3389/fnhum.2016.00120
The Oscillopathic Nature of Language Deficits in Autism: From Genes to Language Evolution
Abstract
Autism spectrum disorders (ASD) are pervasive neurodevelopmental disorders involving a number of deficits to linguistic cognition. The gap between genetics and the pathophysiology of ASD remains open, in particular regarding its distinctive linguistic profile. The goal of this article is to attempt to bridge this gap, focusing on how the autistic brain processes language, particularly through the perspective of brain rhythms. Due to the phenomenon of pleiotropy, which may take some decades to overcome, we believe that studies of brain rhythms, which are not faced with problems of this scale, may constitute a more tractable route to interpreting language deficits in ASD and eventually other neurocognitive disorders. Building on recent attempts to link neural oscillations to certain computational primitives of language, we show that interpreting language deficits in ASD as oscillopathic traits is a potentially fruitful way to construct successful endophenotypes of this condition. Additionally, we will show that candidate genes for ASD are overrepresented among the genes that played a role in the evolution of language. These genes include (and are related to) genes involved in brain rhythmicity. We hope that the type of steps taken here will additionally lead to a better understanding of the comorbidity, heterogeneity, and variability of ASD, and may help achieve a better treatment of the affected populations.
Keywords: RUNX2; autism; biolinguistics; evo-devo; language evolution; neural oscillations.
Figures
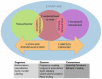

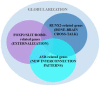

References
-
- Alamri S., Zhang E., Theofanopoulou C., Castillo G., Shi E., Martins P. T., et al. (2015). Implications of subcortical structures in aphasia. Frontiers in Psychology Conference Abstract: Academy of Aphasia 53rd Annual Meeting. Tucson, USA 10.3389/conf.fpsyg.2015.65.00081 - DOI
-
- Balari S., Lorenzo G. (2015). It is an organ, it is new, it is not a new organ. Conceptualizing language from a homological perspective. Front. Ecol. Evol. 3:58 10.3389/fevo.2015.00058 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

