Dual Acting Neuraminidase Inhibitors Open New Opportunities to Disrupt the Lethal Synergism between Streptococcus pneumoniae and Influenza Virus
- PMID: 27047471
- PMCID: PMC4800182
- DOI: 10.3389/fmicb.2016.00357
Dual Acting Neuraminidase Inhibitors Open New Opportunities to Disrupt the Lethal Synergism between Streptococcus pneumoniae and Influenza Virus
Abstract
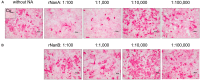
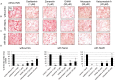
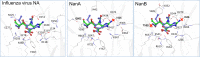
Secondary infections with Streptococcus pneumoniae cause severe pneumonia and enhance lethality during influenza epidemics and pandemics. Structural and functional similarities with viral neuraminidase (NA) suggest that the highly prevalent pneumococcal NAs, NanA and NanB, might contribute to this lethal synergism by supporting viral replication and that dual acting NA inhibitors (NAIs) will disrupt it. To verify this hypothesis, NanA and NanB were expressed in E. coli. After confirming their activity in enzyme assays, in vitro models with influenza virus A/Jena/8178/09 (Jena/8178) and the recombinant NanA or NanB (rNanA and rNanB) were established in A549 and MDCK cells to mimic the role of these pneumococcal NAs during co-infection. Studies on the influence of both NAs on viral receptor expression, spread, and yield revealed a distinct effect of NanA and NanB on viral replication in these in vitro models. Both enzymes were able to support Jena/8178 replication at certain concentrations. This synergism was disrupted by the NAIs oseltamivir, DANA, katsumadain A, and artocarpin exerting an inhibitory effect on viral NA and NanA. Interestingly, katsumadain A and artocarpin inhibited rNanA and rNanB similarly. Zanamivir did not show activity. These results demonstrate a key role of pneumococcal NAs in the lethal synergism with influenza viruses and reveal opportunities for its effective disruption.
Keywords: antibacterial; antiviral; co-pathogenesis; enzyme inhibition; microbial communication; pandemic influenza; pneumococci; secondary pneumococcal pneumonia.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

