B Cell Development in the Bone Marrow Is Regulated by Homeostatic Feedback Exerted by Mature B Cells
- PMID: 27047488
- PMCID: PMC4801882
- DOI: 10.3389/fimmu.2016.00077
B Cell Development in the Bone Marrow Is Regulated by Homeostatic Feedback Exerted by Mature B Cells
Abstract
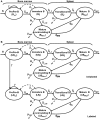
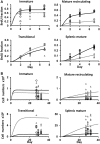
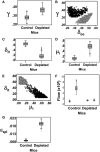
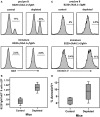
Cellular homeostasis in the B cell compartment is strictly imposed to balance cell production and cell loss. However, it is not clear whether B cell development in the bone marrow is an autonomous process or subjected to regulation by the peripheral B cell compartment. To specifically address this question, we used mice transgenic for human CD20, where effective depletion of B lineage cells is obtained upon administration of mouse anti-human CD20 antibodies, in the absence of any effect on other cell lineages and/or tissues. We followed the kinetics of B cell return to equilibrium by BrdU labeling and flow cytometry and analyzed the resulting data by mathematical modeling. Labeling was much faster in depleted mice. Compared to control mice, B cell-depleted mice exhibited a higher proliferation rate in the pro-/pre-B compartment, and higher cell death and lower differentiation in the immature B cell compartment. We validated the first result by analysis of the expression of Ki67, the nuclear protein expressed in proliferating cells, and the second using Annexin V staining. Collectively, our results suggest that B lymphopoiesis is subjected to homeostatic feedback mechanisms imposed by mature B cells in the peripheral compartment.
Keywords: B lymphocytes; BrdU; computer simulation; homeostatic feedback; mathematical modeling.
Figures


Similar articles
-
A feeder-free differentiation system identifies autonomously proliferating B cell precursors in human bone marrow.J Immunol. 2014 Feb 1;192(3):1044-54. doi: 10.4049/jimmunol.1301815. Epub 2013 Dec 30. J Immunol. 2014. PMID: 24379121
-
Regulation of lymphocyte production in the bone marrow. I. Turnover of small lymphocytes in mice depleted of B lymphocytes by treatment with anti-IgM antibodies.J Immunol. 1983 Feb;130(2):644-8. J Immunol. 1983. PMID: 6600249
-
Chronic B cell deficiency from birth prevents age-related alterations in the B lineage.J Immunol. 2011 Sep 1;187(5):2140-7. doi: 10.4049/jimmunol.1100999. Epub 2011 Aug 1. J Immunol. 2011. PMID: 21810615
-
Bone marrow microenvironmental changes in aged mice compromise V(D)J recombinase activity and B cell generation.Semin Immunol. 2005 Oct;17(5):347-55. doi: 10.1016/j.smim.2005.05.012. Semin Immunol. 2005. PMID: 15963731 Review.
-
Apoptosis and its modulation during B lymphopoiesis in mouse bone marrow.Immunol Rev. 2000 Jun;175:158-74. Immunol Rev. 2000. PMID: 10933601 Review.
Cited by
-
Development of a Mature B Lymphocyte Probe through Gating-Oriented Live-Cell Distinction (GOLD) and Selective Imaging of Topical Spleen.JACS Au. 2024 Feb 26;4(4):1450-1457. doi: 10.1021/jacsau.4c00001. eCollection 2024 Apr 22. JACS Au. 2024. PMID: 38665660 Free PMC article.
-
B cells in non-lymphoid tissues.Nat Rev Immunol. 2025 Jul;25(7):483-496. doi: 10.1038/s41577-025-01137-6. Epub 2025 Feb 5. Nat Rev Immunol. 2025. PMID: 39910240 Review.
-
Coexpression of CCR7 and CXCR4 During B Cell Development Controls CXCR4 Responsiveness and Bone Marrow Homing.Front Immunol. 2019 Dec 18;10:2970. doi: 10.3389/fimmu.2019.02970. eCollection 2019. Front Immunol. 2019. PMID: 31921208 Free PMC article.
-
An Update on the Evolutionary History of Bregs.Genes (Basel). 2022 May 17;13(5):890. doi: 10.3390/genes13050890. Genes (Basel). 2022. PMID: 35627275 Free PMC article. Review.
-
Immune system dynamics in response to Pseudomonas aeruginosa biofilms.NPJ Biofilms Microbiomes. 2025 Jun 12;11(1):104. doi: 10.1038/s41522-025-00738-2. NPJ Biofilms Microbiomes. 2025. PMID: 40506442 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

