Opportunities and challenges for the biodegradable magnesium alloys as next-generation biomaterials
- PMID: 27047673
- PMCID: PMC4817317
- DOI: 10.1093/rb/rbw003
Opportunities and challenges for the biodegradable magnesium alloys as next-generation biomaterials
Abstract
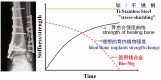
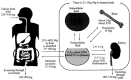
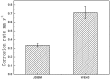
In recent years, biodegradable magnesium alloys emerge as a new class of biomaterials for tissue engineering and medical devices. Deploying biodegradable magnesium-based materials not only avoids a second surgical intervention for implant removal but also circumvents the long-term foreign body effect of permanent implants. However, these materials are often subjected to an uncontrolled and fast degradation, acute toxic responses and rapid structural failure presumably due to a localized, too rapid corrosion process. The patented Mg-Nd-Zn-based alloys (JiaoDa BioMg [JDBM]) have been developed in Shanghai Jiao Tong University in recent years. The alloy series exhibit lower biodegradation rate and homogeneous nanophasic degradation patterns as compared with other biodegradable Mg alloys. The in vitro cytotoxicity tests using various types of cells indicate excellent biocompatibility of JDBM. Finally, bone implants using JDBM-1 alloy and cardiovascular stents using JDBM-2 alloy have been successfully fabricated and in vivo long-term assessment via implantation in animal model have been performed. The results confirmed the reduced degradation rate in vivo, excellent tissue compatibility and long-term structural and mechanical durability. Thus, this novel Mg-alloy series with highly uniform nanophasic biodegradation represent a major breakthrough in the field and a promising candidate for manufacturing the next generation biodegradable implants.
Keywords: biodegradable material; cardiovascular stents; magnesium alloys; orthopaedic implants; uniform corrosion.
Figures





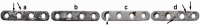
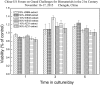

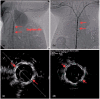
References
-
- Kannan MB, Raman RK. In vitro degradation and mechanical integrity of calcium containing magnesium alloy in modified simulated body fluid. Biomaterials 2008;29:2306–14. - PubMed
-
- Jacobs JJ, Hallab NJ, Skipor AK. et al. Metal degradation products—A cause for concern in metal-metal bearings? Clin Orthop Relat Res 2003;139–47. - PubMed
-
- Puleo DA, Huh WW. Acute toxicity of metal ions in cultures of osteogenic cells derived from bone-marrow stromal cells. J Appl Biomater 1995;6:109–16. - PubMed
-
- Witte F, Kaese V, Haferkamp H. et al. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials 2005;26:3557–63. - PubMed
-
- Witte F, Hort N, Vogt C. et al. Degradable biomaterials based on magnesium corrosion. Current Opin Solid State MaterSci 2008;12:63–72.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

