Glucocorticoids and 11β-HSD1 are major regulators of intramyocellular protein metabolism
- PMID: 27048233
- PMCID: PMC5064767
- DOI: 10.1530/JOE-16-0011
Glucocorticoids and 11β-HSD1 are major regulators of intramyocellular protein metabolism
Abstract
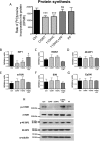
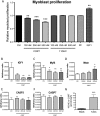
The adverse metabolic effects of prescribed and endogenous glucocorticoid excess, 'Cushing's syndrome', create a significant health burden. While skeletal muscle atrophy and resultant myopathy is a clinical feature, the molecular mechanisms underpinning these changes are not fully defined. We have characterized the impact of glucocorticoids upon key metabolic pathways and processes regulating muscle size and mass including: protein synthesis, protein degradation, and myoblast proliferation in both murine C2C12 and human primary myotube cultures. Furthermore, we have investigated the role of pre-receptor modulation of glucocorticoid availability by 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) in these processes. Corticosterone (CORT) decreased myotube area, decreased protein synthesis, and increased protein degradation in murine myotubes. This was supported by decreased mRNA expression of insulin-like growth factor (IGF1), decreased activating phosphorylation of mammalian target of rapamycin (mTOR), decreased phosphorylation of 4E binding protein 1 (4E-BP1), and increased mRNA expression of key atrophy markers including: atrogin-1, forkhead box O3a (FOXO3a), myostatin (MSTN), and muscle-ring finger protein-1 (MuRF1). These findings were endorsed in human primary myotubes, where cortisol also decreased protein synthesis and increased protein degradation. The effects of 11-dehydrocorticosterone (11DHC) (in murine myotubes) and cortisone (in human myotubes) on protein metabolism were indistinguishable from that of CORT/cortisol treatments. Selective 11β-HSD1 inhibition blocked the decrease in protein synthesis, increase in protein degradation, and reduction in myotube area induced by 11DHC/cortisone. Furthermore, CORT/cortisol, but not 11DHC/cortisone, decreased murine and human myoblast proliferative capacity. Glucocorticoids are potent regulators of skeletal muscle protein homeostasis and myoblast proliferation. Our data underscores the potential use of selective 11β-HSD1 inhibitors to ameliorate muscle-wasting effects associated with glucocorticoid excess.
Keywords: 11β-HSD1; Cushing’s syndrome; glucocorticoid; protein metabolism.
© 2016 Society for Endocrinology.
Figures

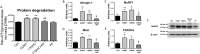

Similar articles
-
Regulation of lipid metabolism by glucocorticoids and 11β-HSD1 in skeletal muscle.Endocrinology. 2013 Jul;154(7):2374-84. doi: 10.1210/en.2012-2214. Epub 2013 Apr 30. Endocrinology. 2013. PMID: 23633532
-
Skeletal muscle 11beta-HSD1 controls glucocorticoid-induced proteolysis and expression of E3 ubiquitin ligases atrogin-1 and MuRF-1.PLoS One. 2011 Jan 31;6(1):e16674. doi: 10.1371/journal.pone.0016674. PLoS One. 2011. PMID: 21304964 Free PMC article.
-
11beta-hydroxysteroid dehydrogenase type 1 regulates glucocorticoid-induced insulin resistance in skeletal muscle.Diabetes. 2009 Nov;58(11):2506-15. doi: 10.2337/db09-0525. Epub 2009 Aug 12. Diabetes. 2009. PMID: 19675138 Free PMC article.
-
Glucocorticoid-induced skeletal muscle atrophy.Int J Biochem Cell Biol. 2013 Oct;45(10):2163-72. doi: 10.1016/j.biocel.2013.05.036. Epub 2013 Jun 24. Int J Biochem Cell Biol. 2013. PMID: 23806868 Review.
-
Tissue-specific glucocorticoid reactivating enzyme, 11 beta-hydroxysteroid dehydrogenase type 1 (11 beta-HSD1)--a promising drug target for the treatment of metabolic syndrome.Curr Drug Targets Immune Endocr Metabol Disord. 2003 Dec;3(4):255-62. doi: 10.2174/1568008033340135. Curr Drug Targets Immune Endocr Metabol Disord. 2003. PMID: 14683456 Review.
Cited by
-
Endogenous Glucocorticoid Signaling in the Regulation of Bone and Marrow Adiposity: Lessons from Metabolism and Cross Talk in Other Tissues.Curr Osteoporos Rep. 2019 Dec;17(6):438-445. doi: 10.1007/s11914-019-00554-6. Curr Osteoporos Rep. 2019. PMID: 31749087 Review.
-
The relationship between temporal muscle thickness and disease activity in Cushing's disease.J Endocrinol Invest. 2023 Nov;46(11):2411-2420. doi: 10.1007/s40618-023-02195-0. Epub 2023 Sep 13. J Endocrinol Invest. 2023. PMID: 37704872
-
Effects of Dexamethasone Dose and Timing on Tissue-Engineered Skeletal Muscle Units.Cells Tissues Organs. 2018;205(4):197-207. doi: 10.1159/000490884. Epub 2018 Aug 17. Cells Tissues Organs. 2018. PMID: 30121672 Free PMC article.
-
The Influence of Post-Exercise Cold-Water Immersion on Adaptive Responses to Exercise: A Review of the Literature.Sports Med. 2018 Jun;48(6):1369-1387. doi: 10.1007/s40279-018-0910-8. Sports Med. 2018. PMID: 29627884 Review.
-
Pathophysiology of Mild Hypercortisolism: From the Bench to the Bedside.Int J Mol Sci. 2022 Jan 8;23(2):673. doi: 10.3390/ijms23020673. Int J Mol Sci. 2022. PMID: 35054858 Free PMC article. Review.
References
-
- Alberts P, Nilsson C, Selen G, Engblom LO, Edling NH, Norling S, Klingstrom G, Larsson C, Forsgren M, Ashkzari M, et al. 2003. Selective inhibition of 11 beta-hydroxysteroid dehydrogenase type 1 improves hepatic insulin sensitivity in hyperglycemic mice strains. Endocrinology 144 4755–4762. (10.1210/en.2003-0344) - DOI - PubMed
-
- Bhat BG, Hosea N, Fanjul A, Herrera J, Chapman J, Thalacker F, Stewart PM, Rejto PA. 2008. Demonstration of proof of mechanism and pharmacokinetics and pharmacodynamic relationship with 4′-cyano-biphenyl-4-sulfonic acid (6-amino-pyridin-2-yl)-amide (PF-915275), an inhibitor of 11-hydroxysteroid dehydrogenase type 1, in cynomolgus monkeys. Journal of Pharmacology and Experimental Therapeutics 324 299–305. (10.1124/jpet.107.128280) - DOI - PubMed
-
- Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, et al. 2001. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nature Cell Biology 3 1014–1019. (10.1038/ncb1101-1014) - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MC_PC_15046/MRC_/Medical Research Council/United Kingdom
- G0502165/MRC_/Medical Research Council/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- MR/K015176/2/MRC_/Medical Research Council/United Kingdom
- BB/G023468/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- G0801473/MRC_/Medical Research Council/United Kingdom
- MC_PC_13066/MRC_/Medical Research Council/United Kingdom
- MR/K015184/1/MRC_/Medical Research Council/United Kingdom
- MC_PC_14109/MRC_/Medical Research Council/United Kingdom
- G0801681/MRC_/Medical Research Council/United Kingdom
- MR/K015176/1/MRC_/Medical Research Council/United Kingdom
- MC_G0802521/MRC_/Medical Research Council/United Kingdom
- MC_G0802531/MRC_/Medical Research Council/United Kingdom
- G0100729/MRC_/Medical Research Council/United Kingdom
- MR/K00414X/1/MRC_/Medical Research Council/United Kingdom
- BBB/S/M/2006/13045 /BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- MC_PC_15055/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

