Oryza sativa H+-ATPase (OSA) is Involved in the Regulation of Dumbbell-Shaped Guard Cells of Rice
- PMID: 27048369
- PMCID: PMC4904443
- DOI: 10.1093/pcp/pcw070
Oryza sativa H+-ATPase (OSA) is Involved in the Regulation of Dumbbell-Shaped Guard Cells of Rice
Abstract
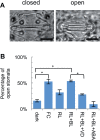
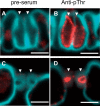
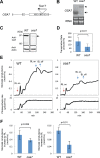
The stomatal apparatus consists of a pair of guard cells and regulates gas exchange between the leaf and atmosphere. In guard cells, blue light (BL) activates H(+)-ATPase in the plasma membrane through the phosphorylation of its penultimate threonine, mediating stomatal opening. Although this regulation is thought to be widely adopted among kidney-shaped guard cells in dicots, the molecular basis underlying that of dumbbell-shaped guard cells in monocots remains unclear. Here, we show that H(+)-ATPases are involved in the regulation of dumbbell-shaped guard cells. Stomatal opening of rice was promoted by the H(+)-ATPase activator fusicoccin and by BL, and the latter was suppressed by the H(+)-ATPase inhibitor vanadate. Using H(+)-ATPase antibodies, we showed the presence of phosphoregulation of the penultimate threonine in Oryza sativa H(+)-ATPases (OSAs) and localization of OSAs in the plasma membrane of guard cells. Interestingly, we identified one H(+)-ATPase isoform, OSA7, that is preferentially expressed among the OSA genes in guard cells, and found that loss of function of OSA7 resulted in partial insensitivity to BL. We conclude that H(+)-ATPase is involved in BL-induced stomatal opening of dumbbell-shaped guard cells in monocotyledon species.
Keywords: Dumbbell-type stomata; H+-ATPase; Light-induced stomatal opening; Rice.
© The Author 2016. Published by Oxford University Press on behalf of Japanese Society of Plant Physiologists.
Figures



Similar articles
-
Immunohistochemical detection of blue light-induced phosphorylation of the plasma membrane H+-ATPase in stomatal guard cells.Plant Cell Physiol. 2011 Jul;52(7):1238-48. doi: 10.1093/pcp/pcr072. Epub 2011 Jun 10. Plant Cell Physiol. 2011. PMID: 21666226
-
Red Light-Induced Phosphorylation of Plasma Membrane H+-ATPase in Stomatal Guard Cells.Plant Physiol. 2018 Oct;178(2):838-849. doi: 10.1104/pp.18.00544. Epub 2018 Aug 13. Plant Physiol. 2018. PMID: 30104254 Free PMC article.
-
Fluence rate dependence of red light-induced phosphorylation of plasma membrane H+-ATPase in stomatal guard cells.Plant Signal Behav. 2019;14(2):1561107. doi: 10.1080/15592324.2018.1561107. Epub 2019 Jan 2. Plant Signal Behav. 2019. PMID: 30601076 Free PMC article.
-
New insights into the regulation of stomatal opening by blue light and plasma membrane H(+)-ATPase.Int Rev Cell Mol Biol. 2011;289:89-115. doi: 10.1016/B978-0-12-386039-2.00003-1. Int Rev Cell Mol Biol. 2011. PMID: 21749899 Review.
-
Protein phosphorylation in stomatal movement.Plant Signal Behav. 2014;9(11):e972845. doi: 10.4161/15592316.2014.972845. Plant Signal Behav. 2014. PMID: 25482764 Free PMC article. Review.
Cited by
-
Plasma membrane-localized H+-ATPase OsAHA3 functions in saline-alkaline stress tolerance in rice.Plant Cell Rep. 2023 Dec 22;43(1):9. doi: 10.1007/s00299-023-03103-9. Plant Cell Rep. 2023. PMID: 38133824
-
Plasma Membrane H+-ATPase SmPHA4 Negatively Regulates the Biosynthesis of Tanshinones in Salvia miltiorrhiza.Int J Mol Sci. 2021 Mar 25;22(7):3353. doi: 10.3390/ijms22073353. Int J Mol Sci. 2021. PMID: 33805926 Free PMC article.
-
Overexpression of Plasma Membrane H+-ATPase in Guard Cells Enhances Light-Induced Stomatal Opening, Photosynthesis, and Plant Growth in Hybrid Aspen.Front Plant Sci. 2021 Nov 26;12:766037. doi: 10.3389/fpls.2021.766037. eCollection 2021. Front Plant Sci. 2021. PMID: 34899787 Free PMC article.
-
Phosphorylation of plasma membrane H+-ATPase Thr881 participates in light-induced stomatal opening.Nat Commun. 2024 Feb 20;15(1):1194. doi: 10.1038/s41467-024-45248-5. Nat Commun. 2024. PMID: 38378616 Free PMC article.
-
Plasma membrane H+-ATPase overexpression increases rice yield via simultaneous enhancement of nutrient uptake and photosynthesis.Nat Commun. 2021 Feb 2;12(1):735. doi: 10.1038/s41467-021-20964-4. Nat Commun. 2021. PMID: 33531490 Free PMC article.
References
-
- Arango M., Gévaudant F., Oufattole M., Boutry M. (2003) The plasma membrane proton pump ATPase: the significance of gene subfamilies. Planta 216: 355–365. - PubMed
-
- Assmann S.M., Grantz D.A. (1990) Stomatal response to humidity in sugarcane and soybean: effect of vapour pressure difference on the kinetics of the blue light response. Plant Cell Environ. 13: 163–169.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

