Generation of Transgenic Porcine Fibroblast Cell Lines Using Nanomagnetic Gene Delivery Vectors
- PMID: 27048425
- PMCID: PMC4829627
- DOI: 10.1007/s12033-016-9934-1
Generation of Transgenic Porcine Fibroblast Cell Lines Using Nanomagnetic Gene Delivery Vectors
Abstract
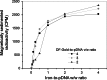
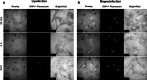
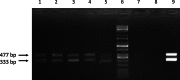
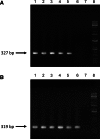
The transgenic process allows for obtaining genetically modified animals for divers biomedical applications. A number of transgenic animals for xenotransplantation have been generated with the somatic cell nuclear transfer (SCNT) method. Thereby, efficient nucleic acid delivery to donor cells such as fibroblasts is of particular importance. The objective of this study was to establish stable transgene expressing porcine fetal fibroblast cell lines using magnetic nanoparticle-based gene delivery vectors under a gradient magnetic field. Magnetic transfection complexes prepared by self-assembly of suitable magnetic nanoparticles, plasmid DNA, and an enhancer under an inhomogeneous magnetic field enabled the rapid and efficient delivery of a gene construct (pCD59-GFPBsd) into porcine fetal fibroblasts. The applied vector dose was magnetically sedimented on the cell surface within 30 min as visualized by fluorescence microscopy. The PCR and RT-PCR analysis confirmed not only the presence but also the expression of transgene in all magnetofected transgenic fibroblast cell lines which survived antibiotic selection. The cells were characterized by high survival rates and proliferative activities as well as correct chromosome number. The developed nanomagnetic gene delivery formulation proved to be an effective tool for the production of genetically engineered fibroblasts and may be used in future in SCNT techniques for breeding new transgenic animals for the purpose of xenotransplantation.
Keywords: Magnetic nanoparticles; Magnetofection; Nucleic acid delivery; Porcine fetal fibroblasts; Transgenesis.
Figures



Similar articles
-
Production of transgenic cloned piglets from genetically transformed fetal fibroblasts selected by green fluorescent protein.Theriogenology. 2005 Mar 1;63(4):973-91. doi: 10.1016/j.theriogenology.2004.04.017. Theriogenology. 2005. PMID: 15710186
-
Development of porcine transgenic nuclear-transferred embryos derived from fibroblast cells transfected by the novel technique of nucleofection or standard lipofection.Theriogenology. 2008 Jul 15;70(2):248-59. doi: 10.1016/j.theriogenology.2008.04.007. Epub 2008 May 22. Theriogenology. 2008. PMID: 18501417
-
Construction of multiple shRNAs expression vector that inhibits FUT1 gene expression and production of the transgenic SCNT embryos in vitro.Mol Biol Rep. 2013 Mar;40(3):2243-52. doi: 10.1007/s11033-012-2287-3. Epub 2012 Dec 1. Mol Biol Rep. 2013. PMID: 23203408
-
Production of transgenic canine embryos using interspecies somatic cell nuclear transfer.Zygote. 2012 Feb;20(1):67-72. doi: 10.1017/S0967199410000651. Epub 2011 Feb 8. Zygote. 2012. PMID: 21303585
-
Magnetofection In Vivo by Nanomagnetic Carriers Systemically Administered into the Bloodstream.Pharmaceutics. 2021 Nov 14;13(11):1927. doi: 10.3390/pharmaceutics13111927. Pharmaceutics. 2021. PMID: 34834342 Free PMC article. Review.
Cited by
-
Improved Delivery of CRISPR/Cas9 System Using Magnetic Nanoparticles into Porcine Fibroblast.Mol Biotechnol. 2019 Mar;61(3):173-180. doi: 10.1007/s12033-018-0145-9. Mol Biotechnol. 2019. PMID: 30560399
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

