Quaternary structure of a G-protein-coupled receptor heterotetramer in complex with Gi and Gs
- PMID: 27048449
- PMCID: PMC4822319
- DOI: 10.1186/s12915-016-0247-4
Quaternary structure of a G-protein-coupled receptor heterotetramer in complex with Gi and Gs
Abstract
Background: G-protein-coupled receptors (GPCRs), in the form of monomers or homodimers that bind heterotrimeric G proteins, are fundamental in the transfer of extracellular stimuli to intracellular signaling pathways. Different GPCRs may also interact to form heteromers that are novel signaling units. Despite the exponential growth in the number of solved GPCR crystal structures, the structural properties of heteromers remain unknown.
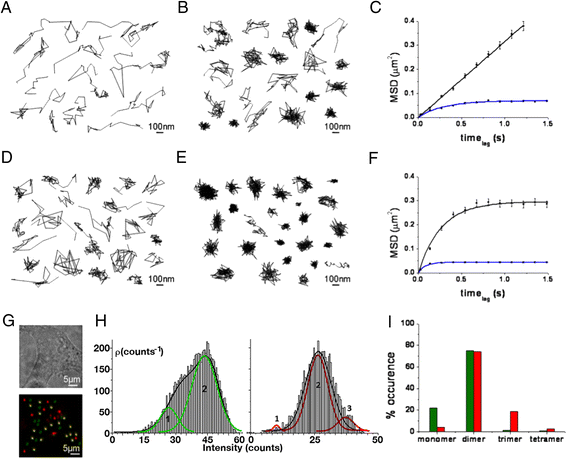
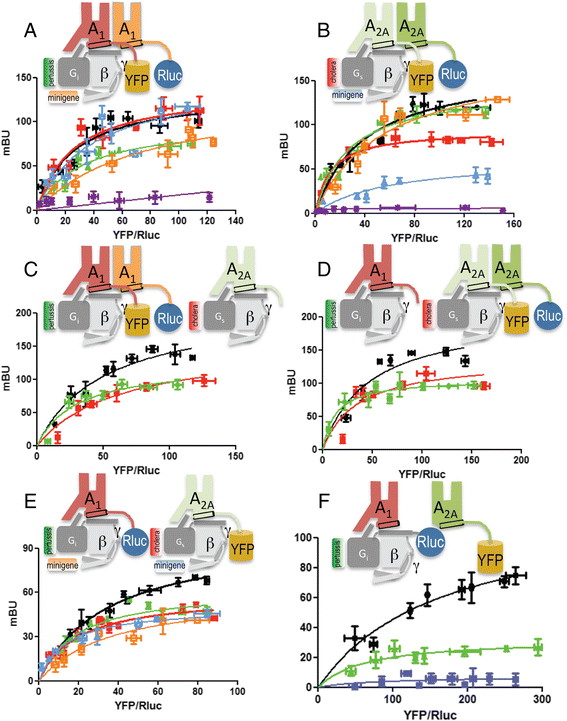
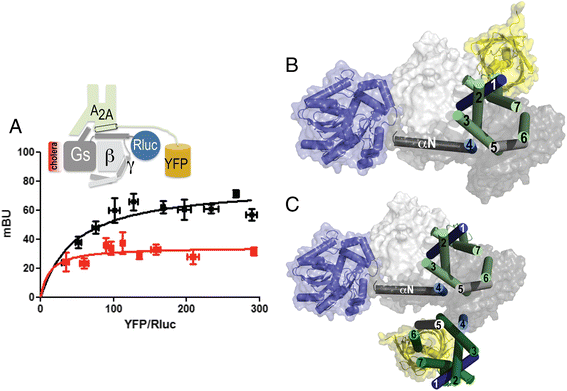
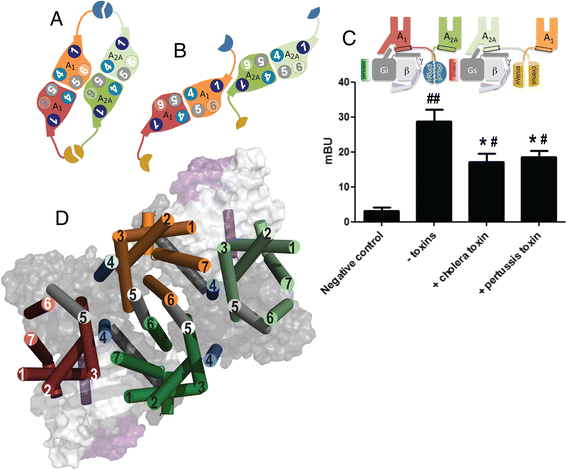
Results: We used single-particle tracking experiments in cells expressing functional adenosine A1-A2A receptors fused to fluorescent proteins to show the loss of Brownian movement of the A1 receptor in the presence of the A2A receptor, and a preponderance of cell surface 2:2 receptor heteromers (dimer of dimers). Using computer modeling, aided by bioluminescence resonance energy transfer assays to monitor receptor homomerization and heteromerization and G-protein coupling, we predict the interacting interfaces and propose a quaternary structure of the GPCR tetramer in complex with two G proteins.
Conclusions: The combination of results points to a molecular architecture formed by a rhombus-shaped heterotetramer, which is bound to two different interacting heterotrimeric G proteins (Gi and Gs). These novel results constitute an important advance in understanding the molecular intricacies involved in GPCR function.
Keywords: BRET; GPCR; Heterotetramer; Heterotrimeric G protein; Molecular modeling; Single-particle tracking.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

