Cysteine S-Glutathionylation Promotes Stability and Activation of the Hippo Downstream Effector Transcriptional Co-activator with PDZ-binding Motif (TAZ)
- PMID: 27048650
- PMCID: PMC4882430
- DOI: 10.1074/jbc.M115.712539
Cysteine S-Glutathionylation Promotes Stability and Activation of the Hippo Downstream Effector Transcriptional Co-activator with PDZ-binding Motif (TAZ)
Abstract
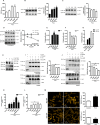
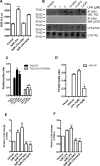
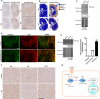
Transcriptional co-activator with PDZ-binding motif (TAZ) and Yes-associated protein (YAP) are critical transcriptional co-activators downstream of the Hippo pathway involved in the regulation of organ size, tissue regeneration, proliferation, and apoptosis. Recent studies suggested common and distinct functions of TAZ and YAP and their diverse impact under several pathological conditions. Here we report differential regulation of TAZ and YAP in response to oxidative stress. H2O2 exposure leads to increased stability and activation of TAZ but not of YAP. H2O2 induces reversible S-glutathionylation at conserved cysteine residues within TAZ. We further demonstrate that TAZ S-glutathionylation is critical for reactive oxygen species (ROS)-mediated, TAZ-dependent TEA domain transcription factor (TEAD) trans-activation. Lysophosphatidic acid, a physiological activator of YAP and TAZ, induces ROS elevation and, subsequently, TAZ S-glutathionylation, which promotes TAZ-mediated target gene expression. TAZ expression is essential for renal homeostasis in mice, and we identify basal TAZ S-glutathionylation in murine kidney lysates, which is elevated during ischemia/reperfusion injury in vivo This induced nuclear localization of TAZ and increased expression of connective tissue growth factor. These results describe a novel mechanism by which ROS sustains total cellular levels of TAZ. This preferential regulation suggests TAZ to be a redox sensor of the Hippo pathway.
Keywords: Hippo pathway; S-glutathionylation; Yes-associated protein (YAP); connective tissue growth factor (CTGF); oxidative stress; reactive oxygen species (ROS); transcriptional co-activator with PDZ-binding motif (TAZ).
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


Similar articles
-
The PDZ-binding motif of Yes-associated protein is required for its co-activation of TEAD-mediated CTGF transcription and oncogenic cell transforming activity.Biochem Biophys Res Commun. 2014 Jan 17;443(3):917-23. doi: 10.1016/j.bbrc.2013.12.100. Epub 2013 Dec 28. Biochem Biophys Res Commun. 2014. PMID: 24380865
-
Proliferation of hepatic stellate cells, mediated by YAP and TAZ, contributes to liver repair and regeneration after liver ischemia-reperfusion injury.Am J Physiol Gastrointest Liver Physiol. 2018 Apr 1;314(4):G471-G482. doi: 10.1152/ajpgi.00153.2017. Epub 2018 Jan 11. Am J Physiol Gastrointest Liver Physiol. 2018. PMID: 29351389 Free PMC article.
-
A novel role for microRNA-129-5p in inhibiting ovarian cancer cell proliferation and survival via direct suppression of transcriptional co-activators YAP and TAZ.Oncotarget. 2015 Apr 20;6(11):8676-86. doi: 10.18632/oncotarget.3254. Oncotarget. 2015. PMID: 25895125 Free PMC article.
-
Reciprocal regulation of YAP/TAZ by the Hippo pathway and the Small GTPase pathway.Small GTPases. 2020 Jul;11(4):280-288. doi: 10.1080/21541248.2018.1435986. Epub 2018 Apr 20. Small GTPases. 2020. PMID: 29457552 Free PMC article. Review.
-
YAP/TAZ for cancer therapy: opportunities and challenges (review).Int J Oncol. 2015 Apr;46(4):1444-52. doi: 10.3892/ijo.2015.2877. Epub 2015 Feb 5. Int J Oncol. 2015. PMID: 25652178 Review.
Cited by
-
YAP/TAZ Signaling and Resistance to Cancer Therapy.Trends Cancer. 2019 May;5(5):283-296. doi: 10.1016/j.trecan.2019.02.010. Epub 2019 Mar 27. Trends Cancer. 2019. PMID: 31174841 Free PMC article. Review.
-
Yes-Associated Protein 1 as a Novel Prognostic Biomarker for Gastrointestinal Cancer: A Meta-Analysis.Biomed Res Int. 2018 Nov 14;2018:4039173. doi: 10.1155/2018/4039173. eCollection 2018. Biomed Res Int. 2018. PMID: 30539010 Free PMC article.
-
Thioredoxin Downregulation Enhances Sorafenib Effects in Hepatocarcinoma Cells.Antioxidants (Basel). 2019 Oct 22;8(10):501. doi: 10.3390/antiox8100501. Antioxidants (Basel). 2019. PMID: 31652503 Free PMC article.
-
ACSL1 Aggravates Thromboinflammation by LPC/LPA Metabolic Axis in Hyperlipidemia Associated Myocardial Ischemia-Reperfusion Injury.Adv Sci (Weinh). 2025 Mar;12(11):e2406359. doi: 10.1002/advs.202406359. Epub 2025 Jan 23. Adv Sci (Weinh). 2025. PMID: 39853712 Free PMC article.
-
Two-edged sword: how activation of the "proto-oncogene" yes-associated protein 1 in lung squamous cell carcinoma can surprisingly inhibit tumor growth.J Thorac Dis. 2018 Nov;10(Suppl 33):S3870-S3874. doi: 10.21037/jtd.2018.10.11. J Thorac Dis. 2018. PMID: 30631502 Free PMC article. No abstract available.
References
-
- Piccolo S., Dupont S., and Cordenonsi M. (2014) The biology of YAP/TAZ: Hippo signaling and beyond. Physiol. Rev. 94, 1287–1312 - PubMed
-
- Wu S., Liu Y., Zheng Y., Dong J., and Pan D. (2008) The TEAD/TEF family protein Scalloped mediates transcriptional output of the Hippo growth-regulatory pathway. Dev. Cell 14, 388–398 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

